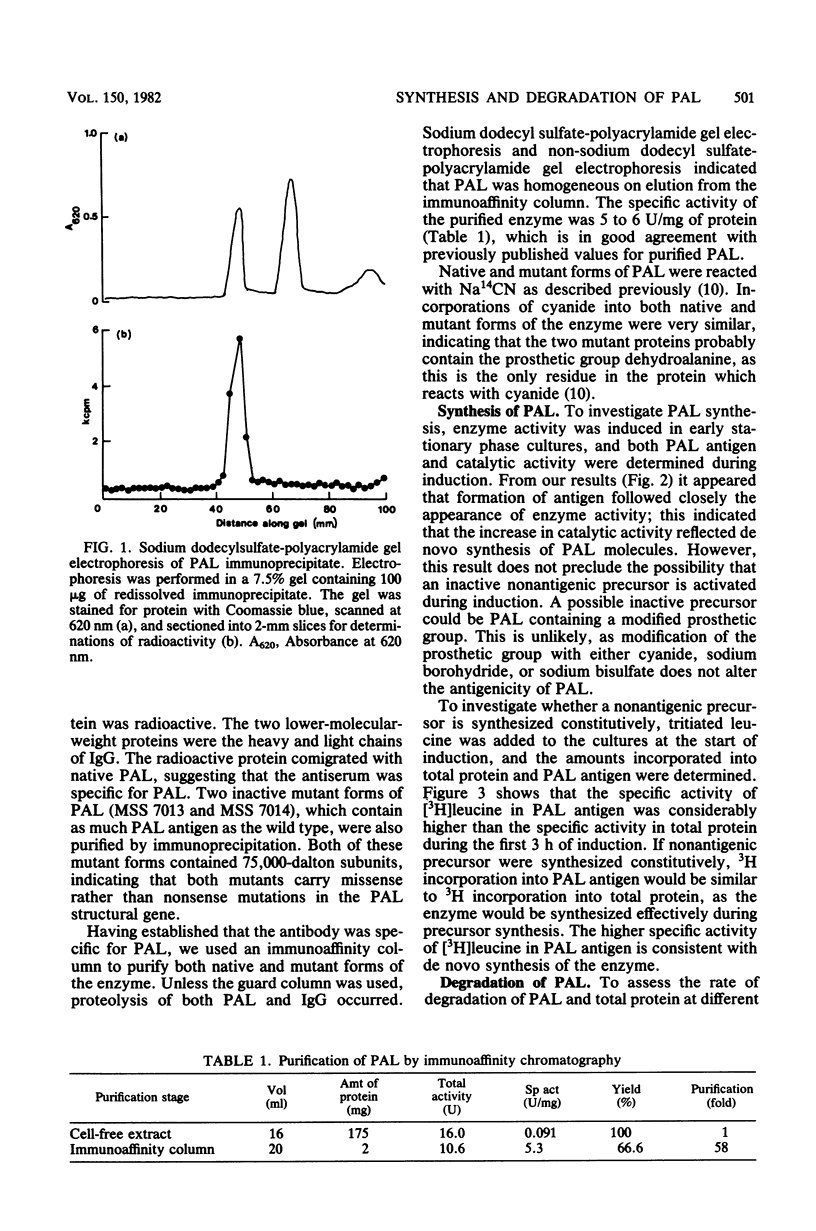
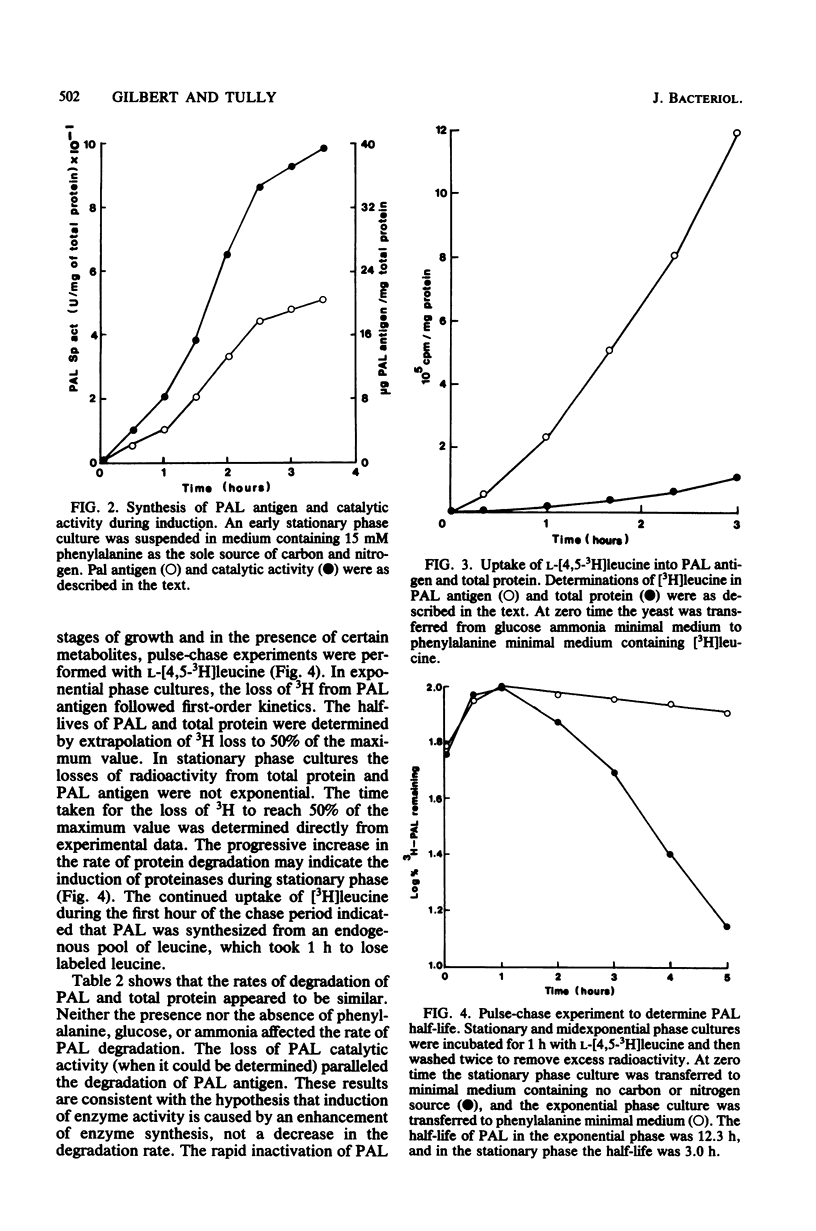
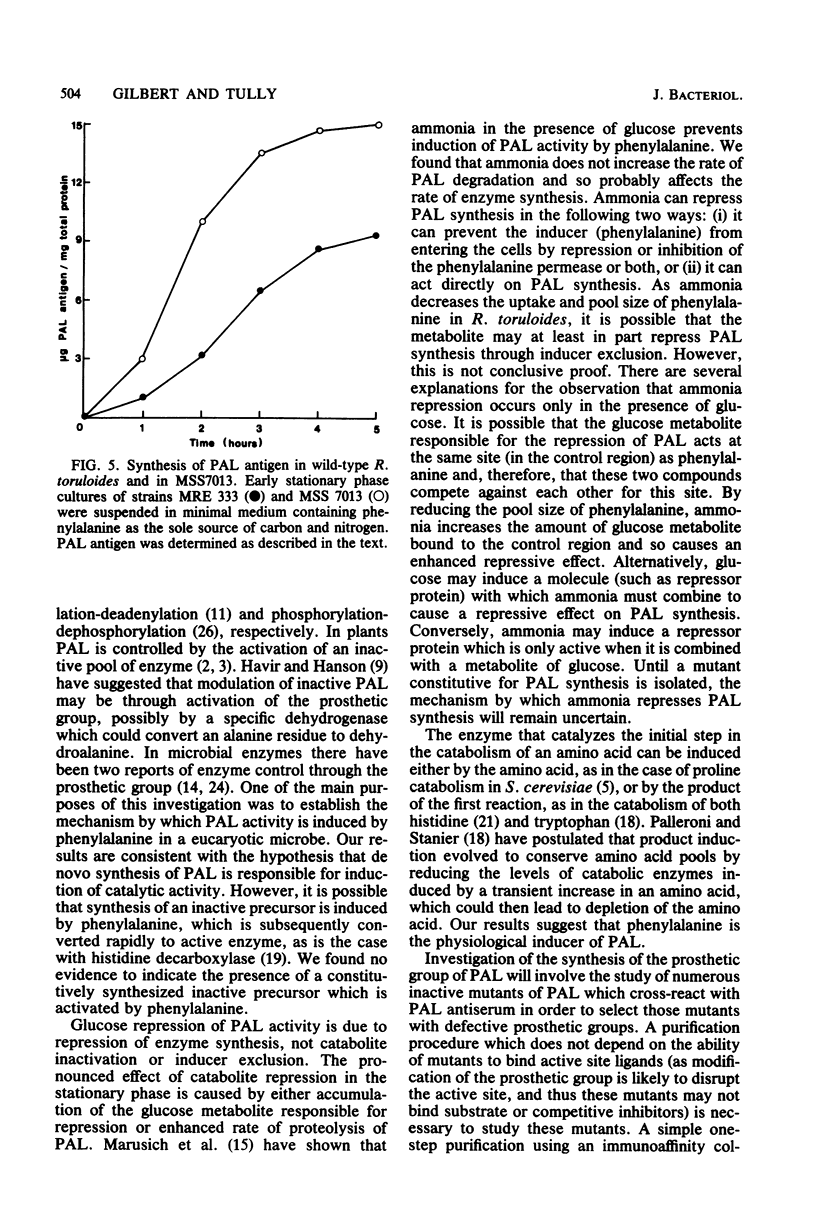
Abstract
The regulation of the enzyme phenylalanine ammonia-lyase (PAL), which is of potential use in oral treatment of phenylketonuria, was investigated. Antiserum against PAL was prepared and was shown to be monospecific for the enzyme by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The native enzyme and two inactive mutant forms of the enzyme were purified to homogeneity by immunoaffinity chromatography, using anti-PAL immunoglobulin G-Sepharose 4B. Both mutant enzymes contained intact prosthetic groups. The formation of PAL catalytic activity after phenylalanine was added to yeast cultures was paralleled by the appearance of enzyme antigen. During induction, uptake of [3H]leucine into the enzyme was higher than uptake into total protein. Our results are consistent with de novo synthesis of an enzyme induced by phenylalanine, rather than activation of a proenzyme. The half-lives of PAL and total protein were similar in both exponential and stationary phase cultures. No metabolite tested affected the rate of enzyme degradation. Glucose repressed enzyme synthesis, whereas ammonia reduced phenylalanine uptake and pool size and so may repress enzyme synthesis through inducer exclusion. The synthesis of enzyme antigen by a mutant unable to metabolize phenylalanine indicated that this amino acid is the physiological inducer of the enzyme.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrhein N., Gerhardt J. Superinduction of phenylalanine ammonia-lyase in gherkin hypocotyls caused by the inhibitor, L-alpha-aminooxy-beta-phenylpropionic acid. Biochim Biophys Acta. 1979 Apr 3;583(4):434–442. doi: 10.1016/0304-4165(79)90060-6. [DOI] [PubMed] [Google Scholar]
- Attridge T. H., Johnson C. B., Smith H. Density-labelling evidence for the phytochrome-mediated activation of phenylalanine ammonia-lyase in mustard cotyledons. Biochim Biophys Acta. 1974 May 24;343(3):440–451. doi: 10.1016/0304-4165(74)90261-x. [DOI] [PubMed] [Google Scholar]
- Attridge T. H., Smith H. Density-labelling evidence for the blue-light-mediated activation of phenylalanine ammonia lyase in Cucumis sativus seedlings. Biochim Biophys Acta. 1974 May 24;343(3):452–464. doi: 10.1016/0304-4165(74)90262-1. [DOI] [PubMed] [Google Scholar]
- Emes A. V., Vining L. C. Partial purification and properties of L-phenylalanine ammonia-lyase from Streptomyces verticillatus. Can J Biochem. 1970 May;48(5):613–622. doi: 10.1139/o70-099. [DOI] [PubMed] [Google Scholar]
- Gilbert H. J., Jack G. W. The effect of proteinases on phenylalanine ammonia-lyase from the yeast Rhodotorula glutinis. Biochem J. 1981 Dec 1;199(3):715–723. doi: 10.1042/bj1990715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havir E. A., Hanson K. R. L-phenylalanine ammonia-lyase. II. Mechanism and kinetic properties of the enzyme from potato tubers. Biochemistry. 1968 May;7(5):1904–1914. doi: 10.1021/bi00845a039. [DOI] [PubMed] [Google Scholar]
- Holzer H., Duntze W. Metabolic regulation by chemical modification of enzymes. Annu Rev Biochem. 1971;40:345–374. doi: 10.1146/annurev.bi.40.070171.002021. [DOI] [PubMed] [Google Scholar]
- Hoskins J. A., Jack G., Wade H. E., Peiris R. J., Wright E. C., Starr D. J., Stern J. Enzymatic control of phenylalanine intake in phenylketonuria. Lancet. 1980 Feb 23;1(8165):392–394. doi: 10.1016/s0140-6736(80)90944-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. Induced biosynthesis of lysine decarboxylase in Bacterium cadaveris. J Gen Microbiol. 1954 Dec;11(3):426–437. doi: 10.1099/00221287-11-3-426. [DOI] [PubMed] [Google Scholar]
- Marusich W. C., Jensen R. A., Zamir L. O. Induction of L-phenylalanine ammonia-lyase during utilization of phenylalanine as a carbon or nitrogen source in Rhodotorula glutinis. J Bacteriol. 1981 Jun;146(3):1013–1019. doi: 10.1128/jb.146.3.1013-1019.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushi N. Iu, Kupletskaia M. B., Bab'eva I. P., Egorov N. S. O fenilalanin-ammiak-liaze pigmentnykh drozhzhei. Mikrobiologiia. 1980 Mar-Apr;49(2):269–273. [PubMed] [Google Scholar]
- PALLERONI N. J., STANIER R. Y. REGULATORY MECHANISMS GOVERNING SYNTHESIS OF THE ENZYMES FOR TRYPTOPHAN OXIDATION BY PSEUDOMONAS FLUORESCENS. J Gen Microbiol. 1964 May;35:319–334. doi: 10.1099/00221287-35-2-319. [DOI] [PubMed] [Google Scholar]
- Rao P. V., Moore K., Towers G. H. Degradation of aromatic amino acids by fungi. II. Purification and properties of phenylalanine ammonia-lyase from Ustilago hordei. Can J Biochem. 1967 Dec;45(12):1863–1872. doi: 10.1139/o67-219. [DOI] [PubMed] [Google Scholar]
- Recsei P. A., Snell E. E. Prohistidine decarboxylase from Lactobacillus 30a. A new type of zymogen. Biochemistry. 1973 Jan 30;12(3):365–371. doi: 10.1021/bi00727a001. [DOI] [PubMed] [Google Scholar]
- Robinson J. H., Anthony C., Drabble W. T. The acidic amino-acid permease of Aspergillus nidulans. J Gen Microbiol. 1973 Nov;79(1):53–63. doi: 10.1099/00221287-79-1-53. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Scotto P., Magasanik B. Exogenous and endogenous induction of the histidine-degrading enzymes in Aerobacter aerogenes. J Biol Chem. 1965 Nov;240(11):4331–4337. [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Switzer R. L. Oxygen-dependent inactivation of glutamine phosphoribosylpyrophosphate amidotransferase in vitro inactivation. J Bacteriol. 1975 Jan;121(1):115–120. doi: 10.1128/jb.121.1.115-120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade H. E., Phillips B. P. Automated determination of bacterial asparaginase and glutaminase. Anal Biochem. 1971 Nov;44(1):189–199. doi: 10.1016/0003-2697(71)90360-5. [DOI] [PubMed] [Google Scholar]
- Wieland O. H., Hartmann U., Siess E. A. Neurospora crassa pyruvate dehydrogenase: interconversion by phosphorylation and dephosphorylation. FEBS Lett. 1972 Nov 1;27(2):240–244. doi: 10.1016/0014-5793(72)80630-6. [DOI] [PubMed] [Google Scholar]



