Abstract
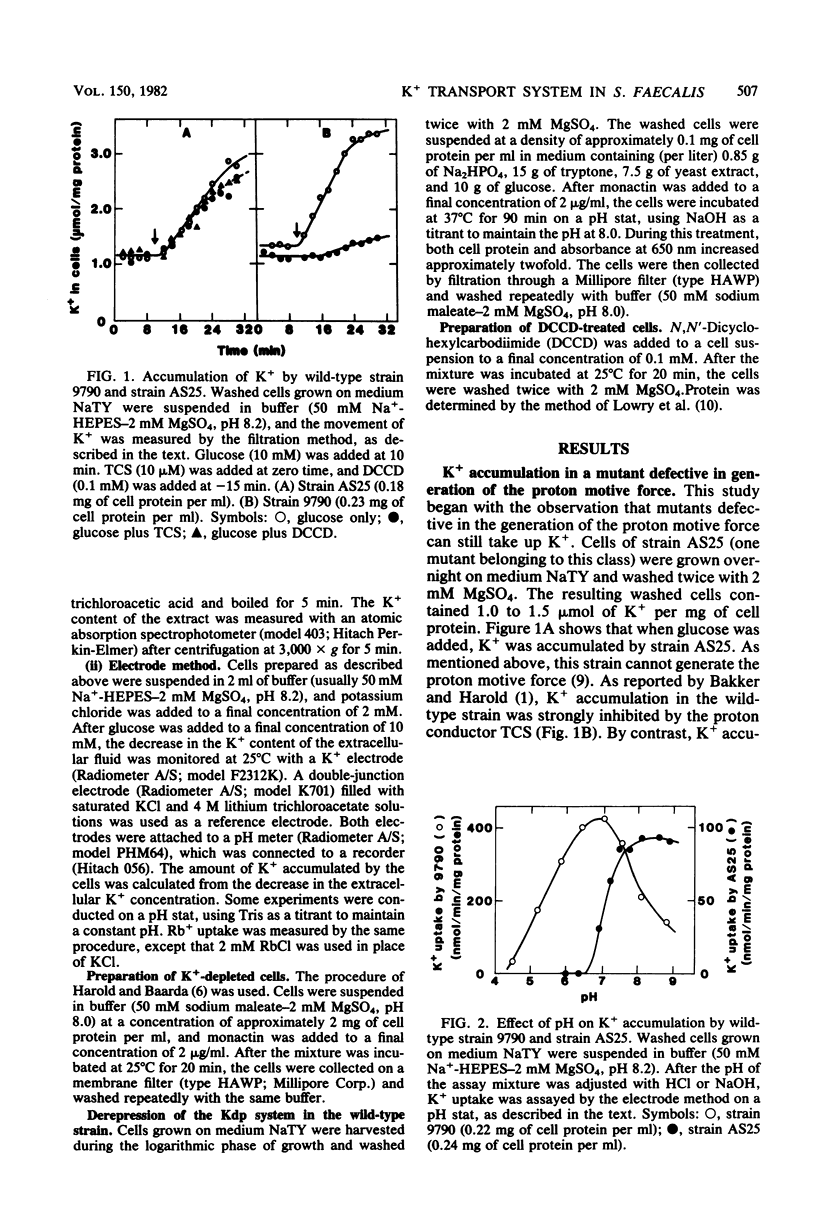
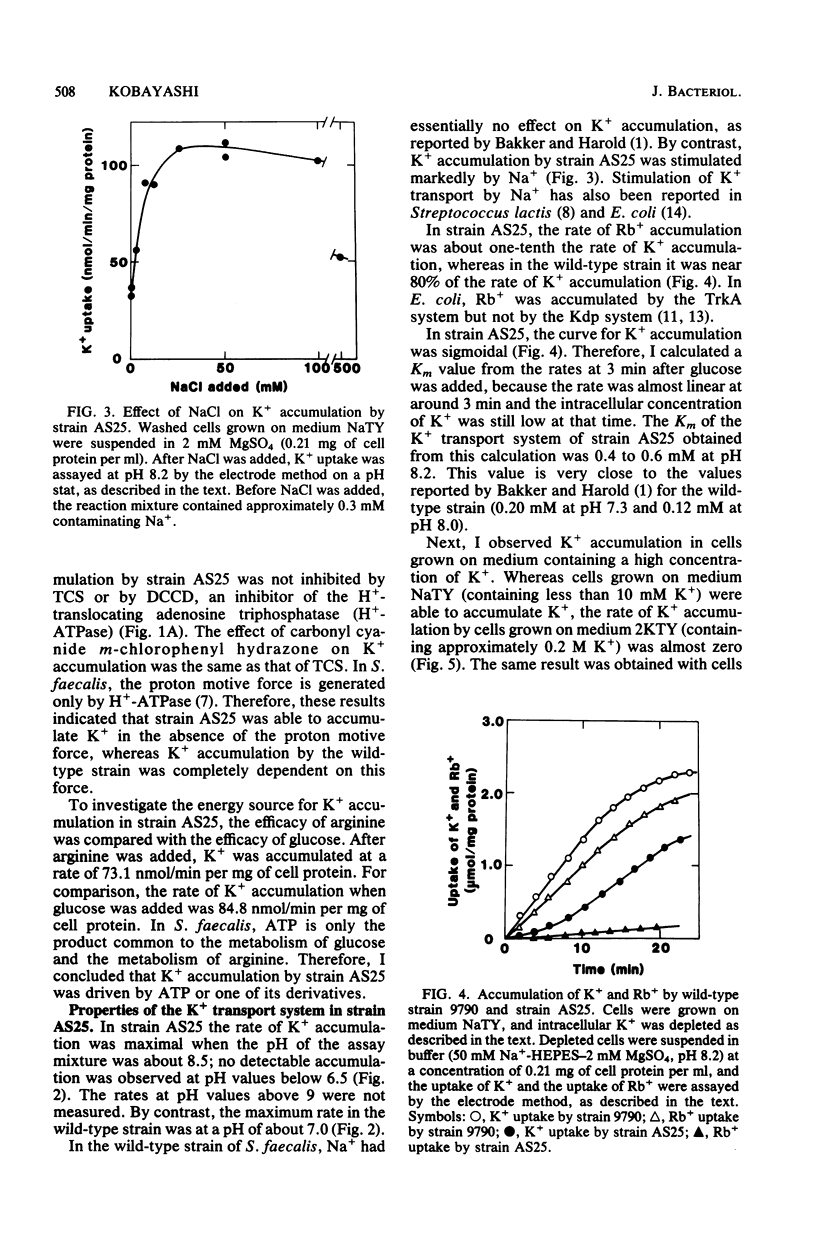
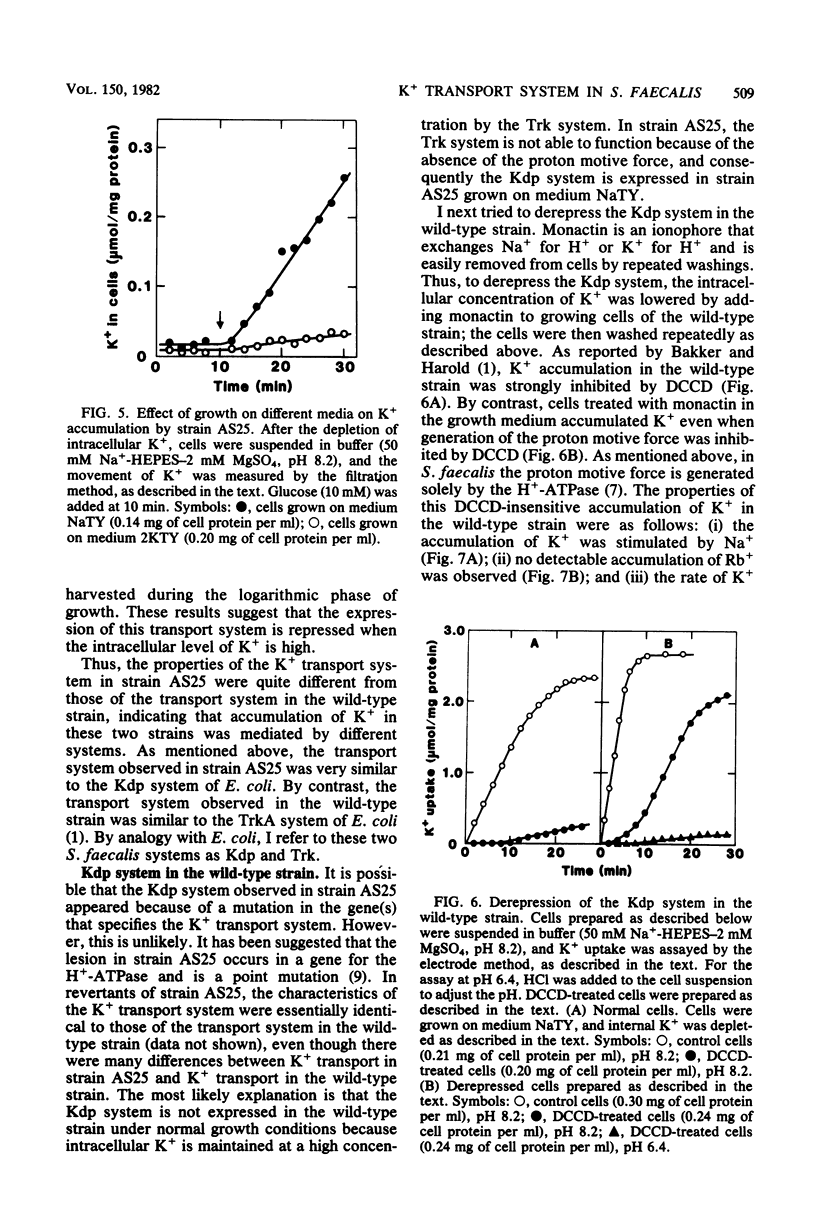
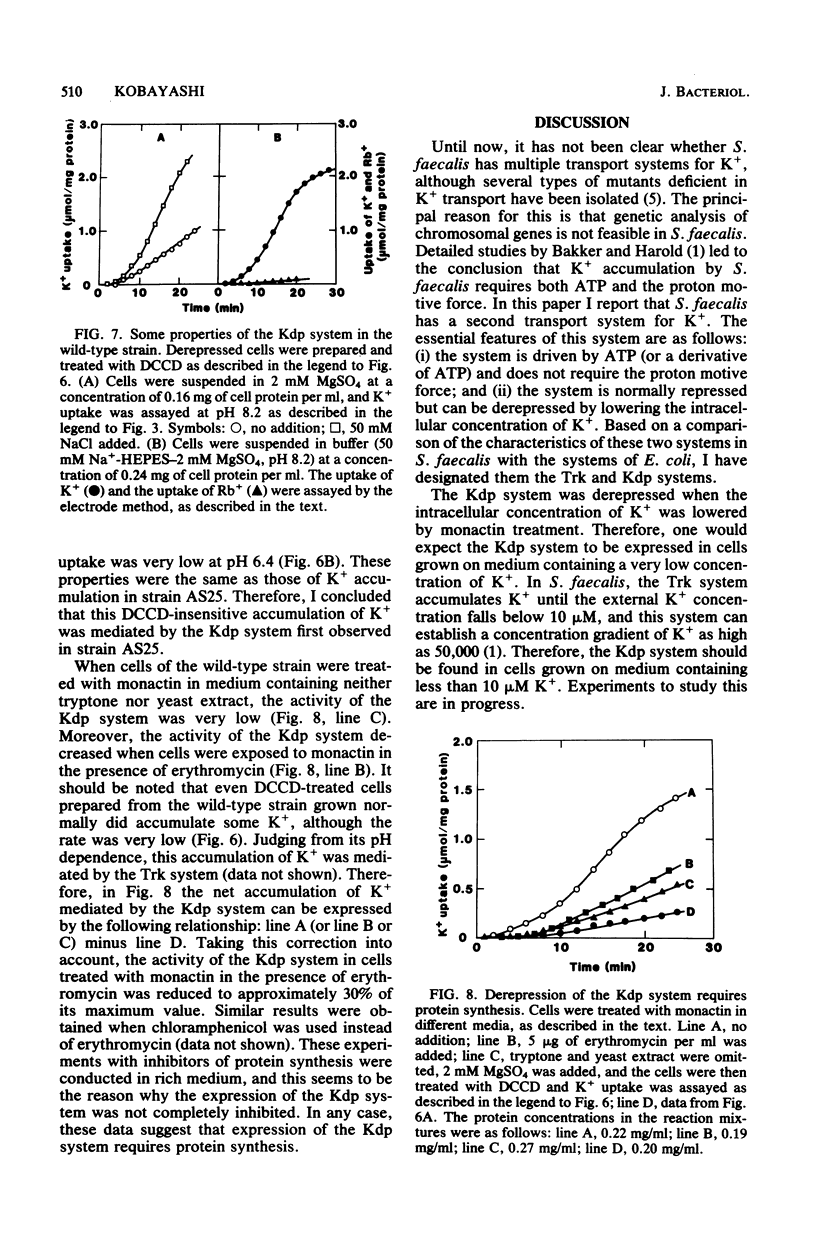
It has been reported that the accumulation of K+ by Streptococcus faecalis is mediated by a transport system which required both ATP and the proton motive force (Bakker and Harold, J. Biol. Chem. 255:433-440, 1980). My results indicate that S. faecalis has a second transport system for K+. The features of this system are as follows: (i) the system is driven by ATP (or a derivative of ATP) and does not require the proton motive force; (ii) the system is normally absent in the wild-type strain but can be derepressed by lowering rhe intracellular concentration of K+; (iii) the pH optimum of this system is about 8.5, and no detectable K+ is accumulated at pH values below 6.5; and (iv) the rate of Rb+ accumulation by this system is very low. These properties are quite different from those of the transport system described by Bakker and Harold. Therefore, I propose that S. faecalis has two K+ transport systems.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker E. P., Harold F. M. Energy coupling to potassium transport in Streptococcus faecalis. Interplay of ATP and the protonmotive force. J Biol Chem. 1980 Jan 25;255(2):433–440. [PubMed] [Google Scholar]
- Erecińska M., Deutsch C. J., Davis J. S. Energy coupling to K+ transport in Paracoccus denitrificans. J Biol Chem. 1981 Jan 10;256(1):278–284. [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Effects of nigericin and monactin on cation permeability of Streptococcus faecalis and metabolic capacities of potassium-depleted cells. J Bacteriol. 1968 Mar;95(3):816–823. doi: 10.1128/jb.95.3.816-823.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Ion currents and physiological functions in microorganisms. Annu Rev Microbiol. 1977;31:181–203. doi: 10.1146/annurev.mi.31.100177.001145. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. II. Proton and sodium extrusion. J Membr Biol. 1972;8(1):45–62. doi: 10.1007/BF01868094. [DOI] [PubMed] [Google Scholar]
- Kashket E. R. Active transport of thallous ions by Streptococcus lactis. J Biol Chem. 1979 Sep 10;254(17):8129–8131. [PubMed] [Google Scholar]
- Kobayashi H., Unemoto T. Streptococcus faecalis mutants defective in regulation of cytoplasmic pH. J Bacteriol. 1980 Sep;143(3):1187–1193. doi: 10.1128/jb.143.3.1187-1193.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nakajima H., Yamato I., Anraku Y. Quantitative analysis of potassium ion pool in Escherichia coli K-12. J Biochem. 1979 Jan;85(1):303–310. doi: 10.1093/oxfordjournals.jbchem.a132325. [DOI] [PubMed] [Google Scholar]
- Rhoads D. B., Epstein W. Energy coupling to net K+ transport in Escherichia coli K-12. J Biol Chem. 1977 Feb 25;252(4):1394–1401. [PubMed] [Google Scholar]
- Rhoads D. B., Woo A., Epstein W. Discrimination between Rb+ and K+ by Escherichia coli. Biochim Biophys Acta. 1977 Aug 15;469(1):45–51. doi: 10.1016/0005-2736(77)90324-8. [DOI] [PubMed] [Google Scholar]
- Sorensen E. N., Rosen B. P. Effects of sodium and lithium ions on the potassium ion transport systems of Escherichia coli. Biochemistry. 1980 Apr 1;19(7):1458–1462. doi: 10.1021/bi00548a030. [DOI] [PubMed] [Google Scholar]


