Abstract
At optimal growth pH (3.0) Thiobacillus acidophilus maintained an internal pH of 5.6 (delta pH of 2.6 units) and a membrane potential (delta psi) of some +73 mV, corresponding to a proton motive force (delta p) of -83 mV. The internal pH remained poised at this value through external pH values of 1 to 5, so that the delta pH increased with decreasing external pH. The positive delta psi increased linearly with delta pH: above a delta pH of 0.6 units, some 60% of the increase in delta pH was compensated for by an opposing increase in delta psi. The highest magnitude of delta pH occurred at an external pH of 1.0, where the cells could not respire. Inhibiting respiration by CN- or azide in cells at optimal pH decreased delta pH by only 0.4 to 0.5 units and caused a corresponding opposite increase in delta psi. Thus, a sizable delta pH could be maintained in the complete absence of respiration. Treatment of cells with thiocyanate to abolish the delta psi resulted in a time-dependent collapse of delta pH, which was augmented by protonophores. We postulate that T. acidophilus possesses unusual resistance to ionic movements. In the presence of a large delta pH (greater than 0.6 pH units), limited diffusion of H+ into the cell is permitted, which generates a positive delta psi because of resistance to compensatory ionic movements. This delta psi, by undergoing fluctuations, regulates the further entry of H+ into the cell in accordance with the metabolic state of the organism. The effect of protonophores was anomalous: the delta p was only partially collapsed, and respiration was strongly inhibited. Possible reasons for this are discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox J. C., Nicholls D. G., Ingledew W. J. Transmembrane electrical potential and transmembrane pH gradient in the acidophile Thiobacillus ferro-oxidans. Biochem J. 1979 Jan 15;178(1):195–200. doi: 10.1042/bj1780195. [DOI] [PMC free article] [PubMed] [Google Scholar]
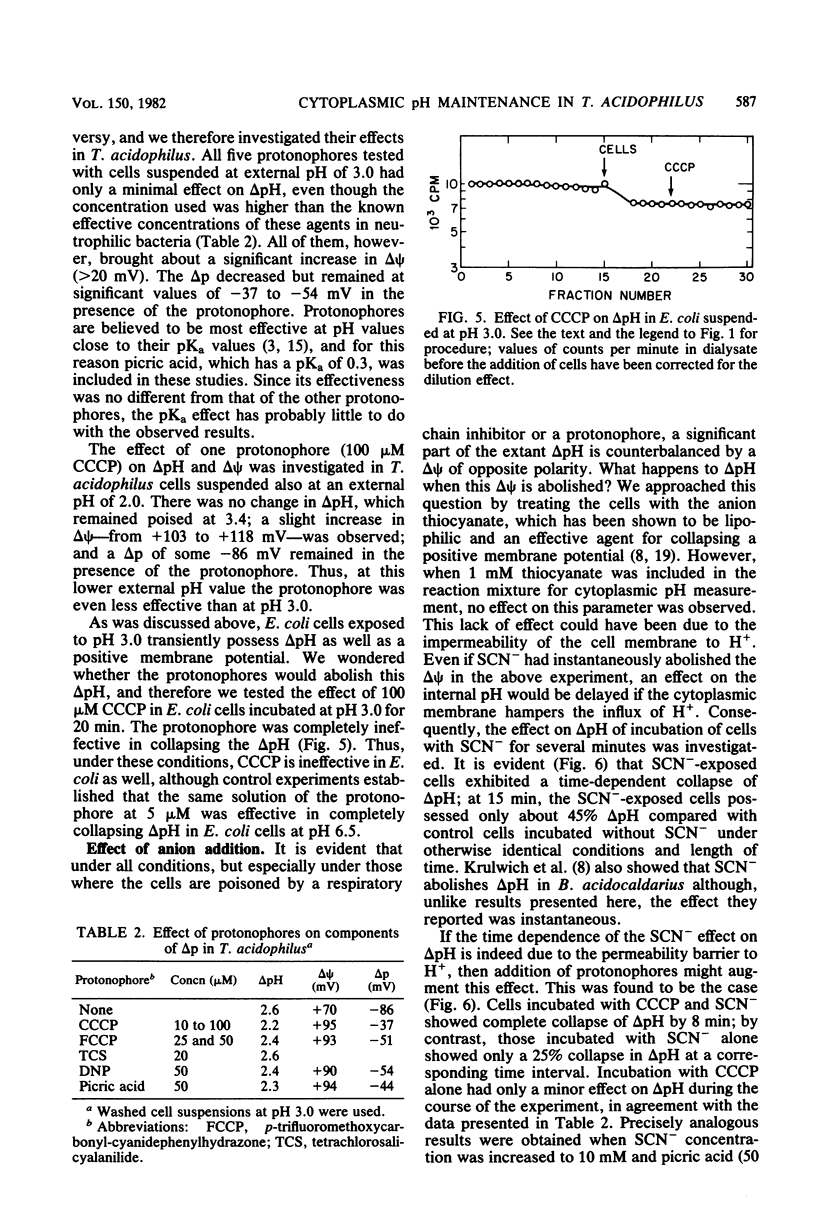
- Feldmann K. New devices for flow dialysis and ultrafiltration for the study of protein--ligand interactions. Anal Biochem. 1978 Jul 15;88(1):225–235. doi: 10.1016/0003-2697(78)90414-1. [DOI] [PubMed] [Google Scholar]
- Finkelstein A. Weak-acid uncouplers of oxidative phosphorylation. Mechanism of action on thin lipid membranes. Biochim Biophys Acta. 1970 Apr 7;205(1):1–6. doi: 10.1016/0005-2728(70)90055-1. [DOI] [PubMed] [Google Scholar]
- Guay R., Silver M. Thiobacillus acidophilus sp. nov.; isolation and some physiological characteristics. Can J Microbiol. 1975 Mar;21(3):281–288. doi: 10.1139/m75-040. [DOI] [PubMed] [Google Scholar]
- Hsung J. C., Haug A. Intracellular pH of Thermoplasma acidophila. Biochim Biophys Acta. 1975 May 21;389(3):477–482. doi: 10.1016/0005-2736(75)90158-3. [DOI] [PubMed] [Google Scholar]
- Hsung J. C., Haug A. Membrane potential of Thermoplasma acidophila. FEBS Lett. 1977 Jan 15;73(1):47–50. doi: 10.1016/0014-5793(77)80011-2. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Davidson L. F., Filip S. J., Jr, Zuckerman R. S., Guffanti A. A. The protonmotive force and beta-galactoside transport in Bacillus acidocaldarius. J Biol Chem. 1978 Jul 10;253(13):4599–4603. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leefeldt R. H., Matin A. Growth and physiology of Thiobacillus novellus under nutrient-limited mixotrophic conditions. J Bacteriol. 1980 May;142(2):645–650. doi: 10.1128/jb.142.2.645-650.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Konings W. N. Transport of lactate and succinate by membrane vesicles of Escherichia coli, Bacillus subtilis and a pseudomonas species. Eur J Biochem. 1973 Apr 2;34(1):58–67. doi: 10.1111/j.1432-1033.1973.tb02728.x. [DOI] [PubMed] [Google Scholar]
- Matin A. Organic nutrition of chemolithotrophic bacteria. Annu Rev Microbiol. 1978;32:433–468. doi: 10.1146/annurev.mi.32.100178.002245. [DOI] [PubMed] [Google Scholar]
- Matin A., Schleiss M., Perez R. C. Regulation of glucose transport and metabolism in Thiobacillus novellus. J Bacteriol. 1980 May;142(2):639–644. doi: 10.1128/jb.142.2.639-644.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Veldhuis C., Stegeman V., Veenhuis M. Selective advantage of a Spirillum sp. in a carbon-limited environment. Accumulation of poly-beta-hydroxybutyric acid and its role in starvation. J Gen Microbiol. 1979 Jun;112(2):349–355. doi: 10.1099/00221287-112-2-349. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Dilger J. P. Transport of protons across membranes by weak acids. Physiol Rev. 1980 Jul;60(3):825–863. doi: 10.1152/physrev.1980.60.3.825. [DOI] [PubMed] [Google Scholar]
- Oshima T., Arakawa H., Baba M. Biochemical studies on an acidophilic, thermophilic bacterium, Bacillus acidocaldarius: isolation of bacteria, intracellular pH, and stabilities of biopolymers. J Biochem. 1977 Apr;81(4):1107–1113. doi: 10.1093/oxfordjournals.jbchem.a131535. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The use of flow dialysis for determinations of deltapH and active transport. Methods Enzymol. 1979;55:680–688. doi: 10.1016/0076-6879(79)55076-9. [DOI] [PubMed] [Google Scholar]
- Romano A. H., Van Vranken N. J., Preisand P., Brustolon M. Regulation of the Thiobacillus intermedius glucose uptake system by thiosulfate. J Bacteriol. 1975 Feb;121(2):577–582. doi: 10.1128/jb.121.2.577-582.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of transmembrane electrochemical proton gradients. J Bioenerg. 1975 May;7(2):61–74. doi: 10.1007/BF01558427. [DOI] [PubMed] [Google Scholar]
- Searcy D. G. Thermoplasma acidophilum: intracellular pH and potassium concentration. Biochim Biophys Acta. 1976 Nov 18;451(1):278–286. doi: 10.1016/0304-4165(76)90278-6. [DOI] [PubMed] [Google Scholar]



