Abstract
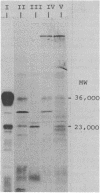
The tetracycline resistance regulatory gene (tetR) of transposon Tn10 was analyzed by a combination of methods involving gene fusion and cloning. This gene is located on a 695-base pair HincII DNA fragment near the center of Tn10. The direction of transcription is opposite to that of neighboring gene tetA, which encodes the TetA protein. The gene product of the tetR gene (the TetR protein) has a molecular weight of 23,000. tet-R-lacZ gene fusions encode fusion beta-galactosidases that are membrane bound, indicating that the TetR protein itself is membrane associated. Mutants defective in tetR result in constitutive tetracycline resistance, but the level of resistance is reduced. Expression of the tetR gene is induced by tetracycline; in the absence of tetracycline, the TetR protein turns off its own synthesis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Manning P. A., Edelbluth C., Herrlich P. Export without proteolytic processing of inner and outer membrane proteins encoded by F sex factor tra cistrons in Escherichia coli minicells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4837–4841. doi: 10.1073/pnas.76.10.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman K., Hawley D., Ross M. J. Use of phage immunity in molecular cloning experiments. Science. 1977 Apr 8;196(4286):182–183. doi: 10.1126/science.847464. [DOI] [PubMed] [Google Scholar]
- Ball P. R., Shales S. W., Chopra I. Plasmid-mediated tetracycline resistance in Escherichia coli involves increased efflux of the antibiotic. Biochem Biophys Res Commun. 1980 Mar 13;93(1):74–81. doi: 10.1016/s0006-291x(80)80247-6. [DOI] [PubMed] [Google Scholar]
- Barnes W. M., Siegel R. B., Reznikoff W. S. The construction of lambda transducing phages containing deletions defining regulatory elements of the lac and trp operons in E. coli. Mol Gen Genet. 1974 Mar 27;129(3):201–215. doi: 10.1007/BF00267913. [DOI] [PubMed] [Google Scholar]
- Beck C. F. A genetic approach to analysis of transposons. Proc Natl Acad Sci U S A. 1979 May;76(5):2376–2380. doi: 10.1073/pnas.76.5.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I., Howe T. G. Bacterial resistance to the tetracyclines. Microbiol Rev. 1978 Dec;42(4):707–724. doi: 10.1128/mr.42.4.707-724.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. C., Foster T. J. Analysis of the reduction in expression of tetracycline resistance determined by transposon Tn10 in the multicopy state. Mol Gen Genet. 1981;182(1):171–177. doi: 10.1007/BF00422786. [DOI] [PubMed] [Google Scholar]
- Franklin T. J., Cook J. M. R factor with a mutation in the tetracycline resistance marker. Nature. 1971 Jan 22;229(5282):273–274. doi: 10.1038/229273a0. [DOI] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Müller-Hill B. Synthetic multifunctional proteins: isolation of covalently linked tryptophan synthetase alpha-subunit-lac-repressor-beta-galactosidase chimeras. Mol Gen Genet. 1977 Oct 24;155(3):301–307. doi: 10.1007/BF00272809. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Reznikoff W. S. Organization of structural and regulatory genes that mediate tetracycline resistance in transposon Tn10. J Bacteriol. 1979 Jun;138(3):705–714. doi: 10.1128/jb.138.3.705-714.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson T. J., Lightner V. A., Green P. R., Modrich P., Bell R. M. Membrane phospholipid synthesis in Escherichia coli. Identification of the sn-glycerol-3-phosphate acyltransferase polypeptide as the plsB gene product. J Biol Chem. 1980 Oct 10;255(19):9421–9426. [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L., Petrucci R. E., Jr, Levy S. B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez B., Tachibana C., Levy S. B. Heterogeneity of tetracycline resistance determinants. Plasmid. 1980 Mar;3(2):99–108. doi: 10.1016/0147-619x(80)90101-8. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Josefsson L. G. Precursors of three exported proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1209–1212. doi: 10.1073/pnas.75.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R. N., Rogers S. G. Plasmid pKC7: a vector containing ten restriction endonuclease sites suitable for cloning DNA segments. Gene. 1979 Sep;7(1):79–82. doi: 10.1016/0378-1119(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Reeve E. C. R plasmid R6-5 retains an active tet protein repressor gene. Plasmid. 1978 Sep;1(4):581–583. doi: 10.1016/0147-619x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Reeve E. C., Robertson J. M. The characteristics of eleven mutants of R-factor R57 constitutive for tetracycline resistance, selected and tested in Escherichia coli K12. Genet Res. 1975 Jun;25(3):297–312. doi: 10.1017/s001667230001572x. [DOI] [PubMed] [Google Scholar]
- Ross D. G., Swan J., Kleckner N. Physical structures of Tn10-promoted deletions and inversions: role of 1400 bp inverted repetitions. Cell. 1979 Apr;16(4):721–731. doi: 10.1016/0092-8674(79)90088-6. [DOI] [PubMed] [Google Scholar]
- Schumacher G., Bussmann K. Cell-free synthesis of proteins related to sn-glycerol-3-phosphate transport in Escherichia coli. J Bacteriol. 1978 Jul;135(1):239–250. doi: 10.1128/jb.135.1.239-250.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann W., Bade E. G. In vitro constructed plasmids containing both ends of bacteriophage Mu DNA express phage functions. Mol Gen Genet. 1979 Jan 16;169(1):97–105. doi: 10.1007/BF00267550. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Starlinger P., Saedler H. IS-elements in microorganisms. Curr Top Microbiol Immunol. 1976;75:111–152. doi: 10.1007/978-3-642-66530-1_4. [DOI] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait R. C., Boyer H. W. On the nature of tetracycline resistance controlled by the plasmid pSC101. Cell. 1978 Jan;13(1):73–81. doi: 10.1016/0092-8674(78)90139-3. [DOI] [PubMed] [Google Scholar]
- Tait R. C., Rodriguez R. L., Boyer H. W. Altered tetracycline resistance in pSC101 recombinant plasmids. Mol Gen Genet. 1977 Mar 16;151(3):327–331. doi: 10.1007/BF00268797. [DOI] [PubMed] [Google Scholar]
- Wray L. V., Jr, Jorgensen R. A., Reznikoff W. S. Identification of the tetracycline resistance promoter and repressor in transposon Tn10. J Bacteriol. 1981 Aug;147(2):297–304. doi: 10.1128/jb.147.2.297-304.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. L., Zubay G., Levy S. B. Synthesis of an R plasmid protein associated with tetracycline resistance is negatively regulated. Proc Natl Acad Sci U S A. 1976 May;73(5):1509–1512. doi: 10.1073/pnas.73.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupancic T. J., King S. R., Pogue-Geile K. L., Jaskunas S. R. Identification of a second tetracycline-inducible polypeptide encoded by Tn10. J Bacteriol. 1980 Oct;144(1):346–355. doi: 10.1128/jb.144.1.346-355.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]