Abstract
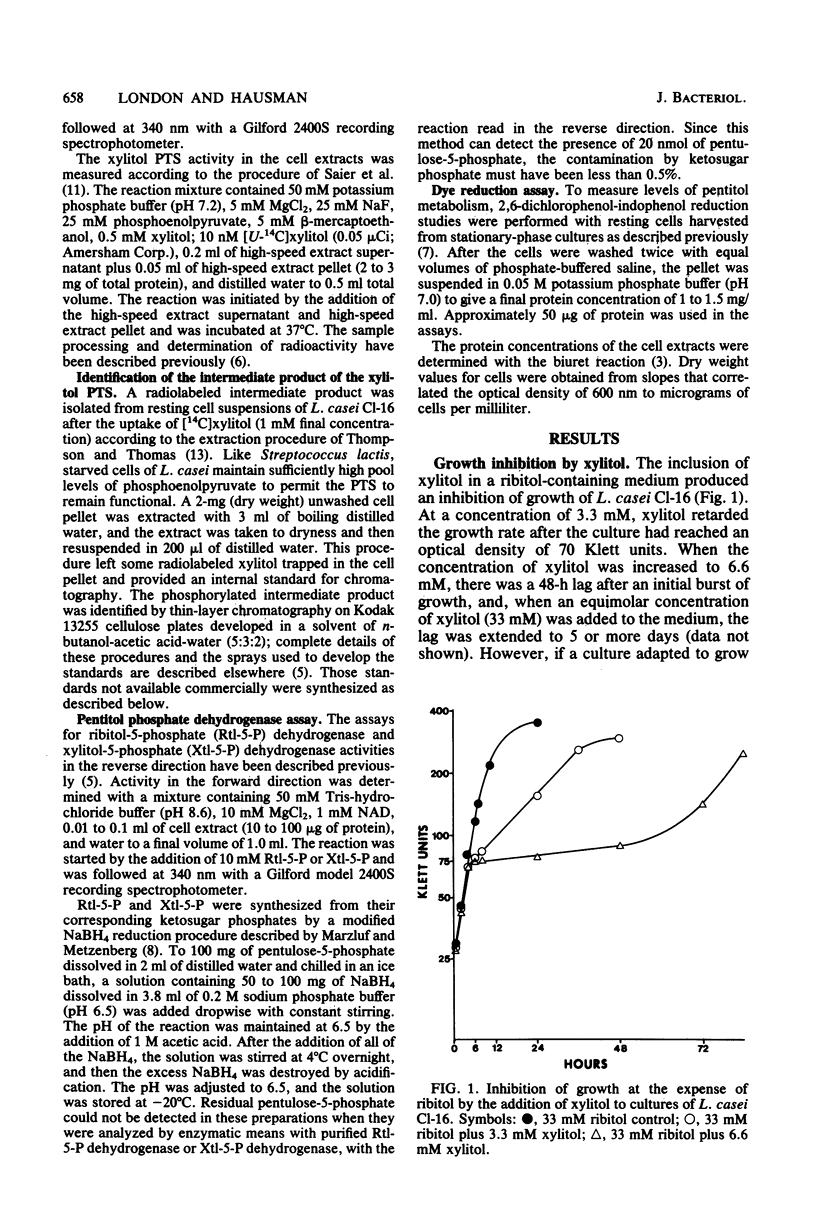
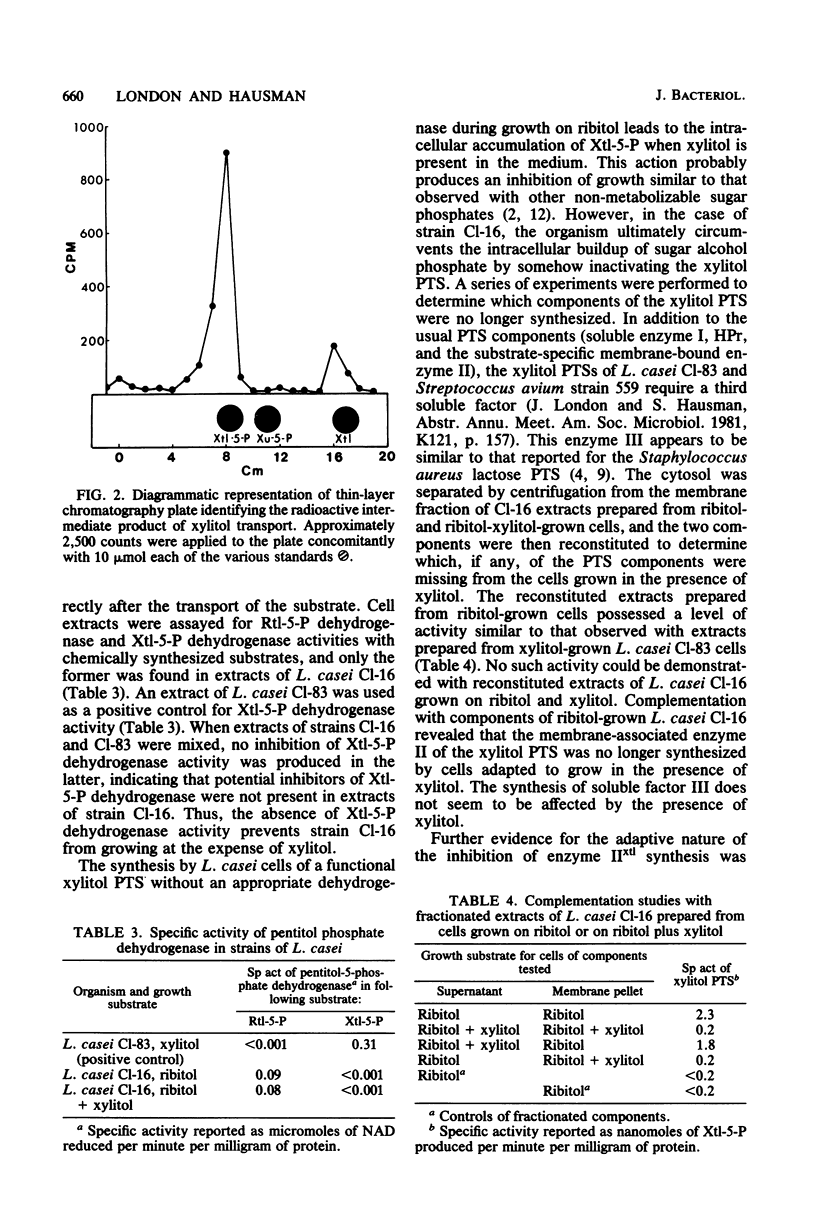
The growth of Lactobacillus casei strain Cl-16 at the expense or ribitol was inhibited if the non-metabolizable substrate xylitol was included in the medium at concentrations of 6 mM or greater. At these concentrations, xylitol, did not competitively inhibit ribitol transport. The cessation of growth was caused by the intracellular accumulation of xylitol-5-phosphate, which occurred because growth on ribitol had gratuitously induced a functional xylitol-specific phosphotransferase system but not the enzymes necessary for the further metabolism of xylitol-5-phosphate. Eventually, the cells overcame the xylitol-mediated inhibition by repressing the synthesis of enzyme II of the xylitol phosphotransferase system so that xylitol-5-phosphate would no longer be accumulated within the cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ENGLESBERG E., ANDERSON R. L., WEINBERG R., LEE N., HOFFEE P., HUTTENHAUER G., BOYER H. L-Arabinose-sensitive, L-ribulose 5-phosphate 4-epimerase-deficient mutants of Escherichia coli. J Bacteriol. 1962 Jul;84:137–146. doi: 10.1128/jb.84.1.137-146.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte T., Hengstenberg W. Purification and characterization of the inducible lactose-specific membrane-bound component of the staphylococcal phosphenolpyruvate-dependent phosphotransferase system. Eur J Biochem. 1971 Nov 11;23(2):295–302. doi: 10.1111/j.1432-1033.1971.tb01621.x. [DOI] [PubMed] [Google Scholar]
- London J., Chace N. M. New pathway for the metabolism of pentitols. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4296–4300. doi: 10.1073/pnas.74.10.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Chace N. M. Pentitol metabolism in Lactobacillus casei. J Bacteriol. 1979 Dec;140(3):949–954. doi: 10.1128/jb.140.3.949-954.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Meyer E. Y. Malate utilization by a group D Streptococcus: physiological properties and purification of an inducible malic enzyme. J Bacteriol. 1969 May;98(2):705–711. doi: 10.1128/jb.98.2.705-711.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T., Simoni R. D., Hays J. B., Roseman S. Phosphorylation of a sugar-specific protein component of the lactose transport system in Staphylococcus aureus. Biochem Biophys Res Commun. 1971 Mar 5;42(5):836–843. doi: 10.1016/0006-291x(71)90506-7. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Simoni R. D., Roseman S. The physiological behavior of enzyme I and heat-stable protein mutants of a bacterial phosphotransferase system. J Biol Chem. 1970 Nov 10;245(21):5870–5873. [PubMed] [Google Scholar]
- St Martin E. J., Wittenberger C. L. Characterization of a phosphoenolpyruvate-dependent sucrose phosphotransferase system in Streptococcus mutans. Infect Immun. 1979 Jun;24(3):865–868. doi: 10.1128/iai.24.3.865-868.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Thomas T. D. Phosphoenolpyruvate and 2-phosphoglycerate: endogenous energy source(s) for sugar accumulation by starved cells of Streptococcus lactis. J Bacteriol. 1977 May;130(2):583–595. doi: 10.1128/jb.130.2.583-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



