Abstract
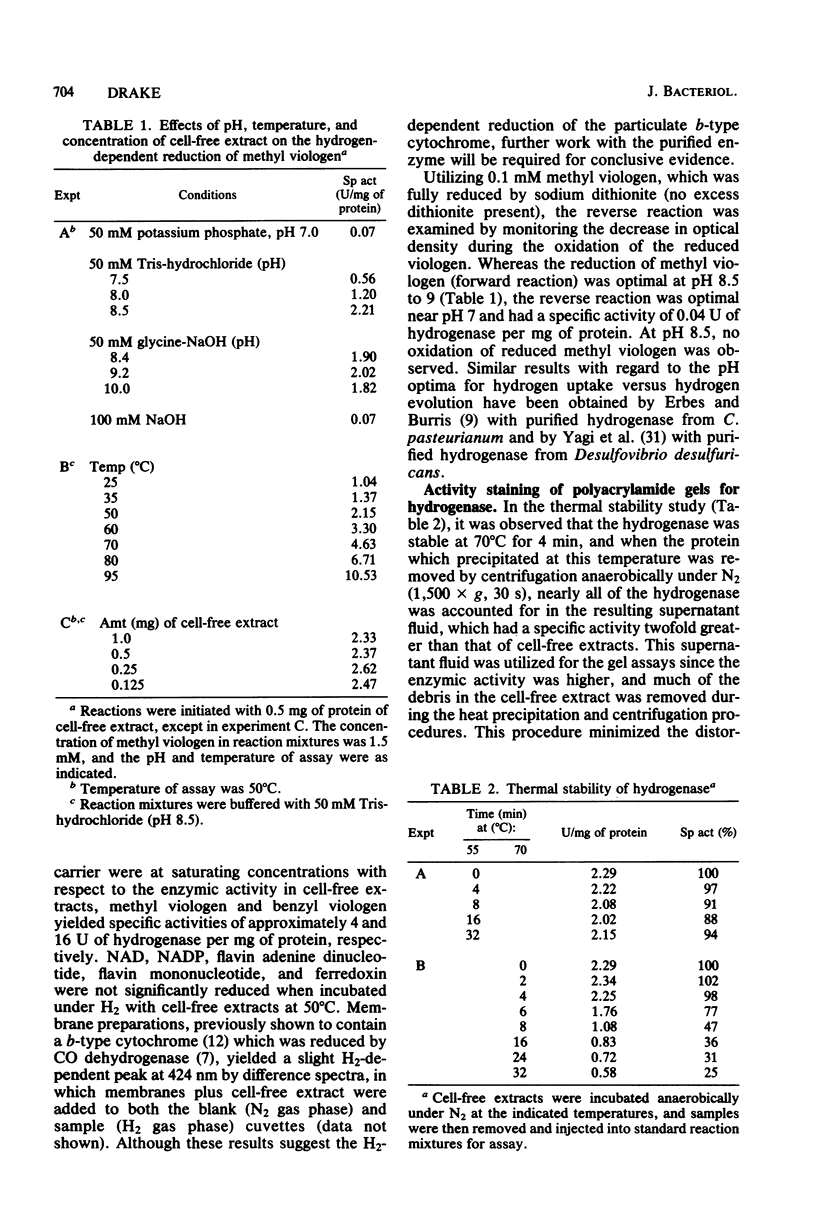
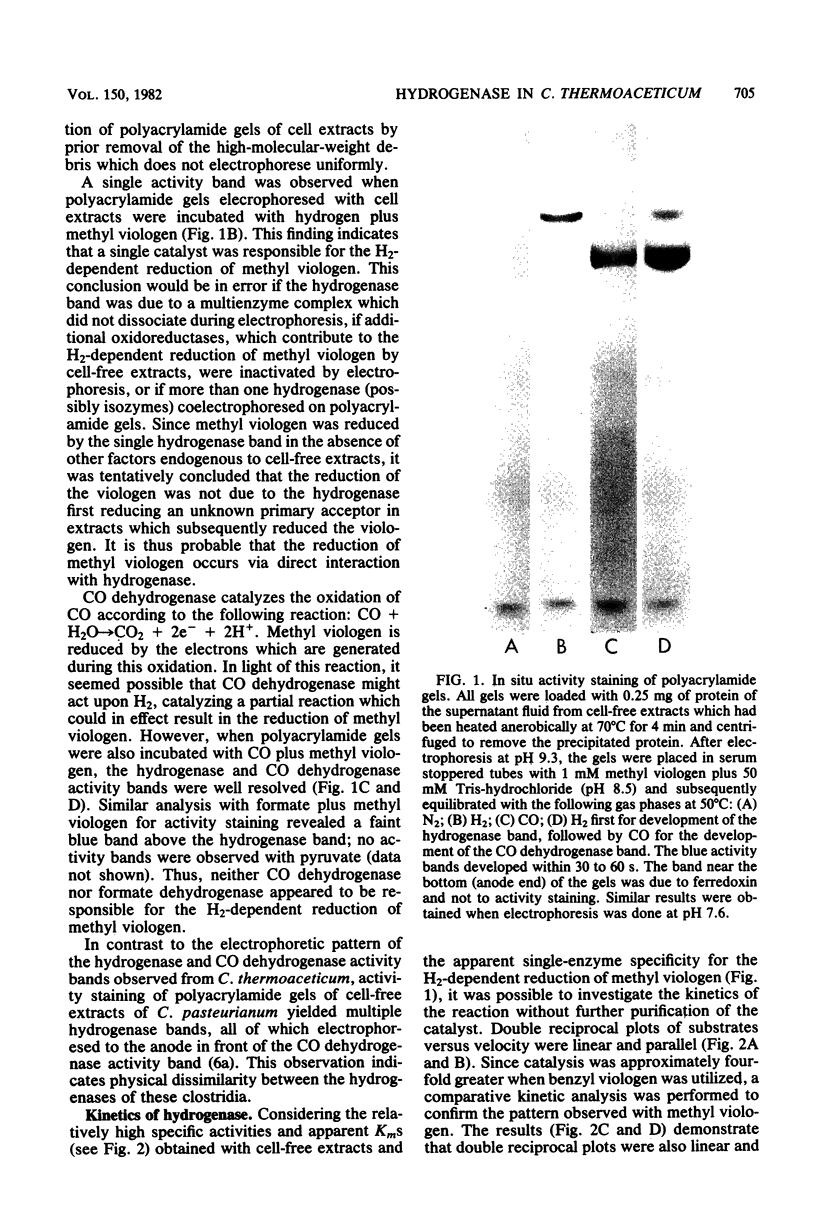
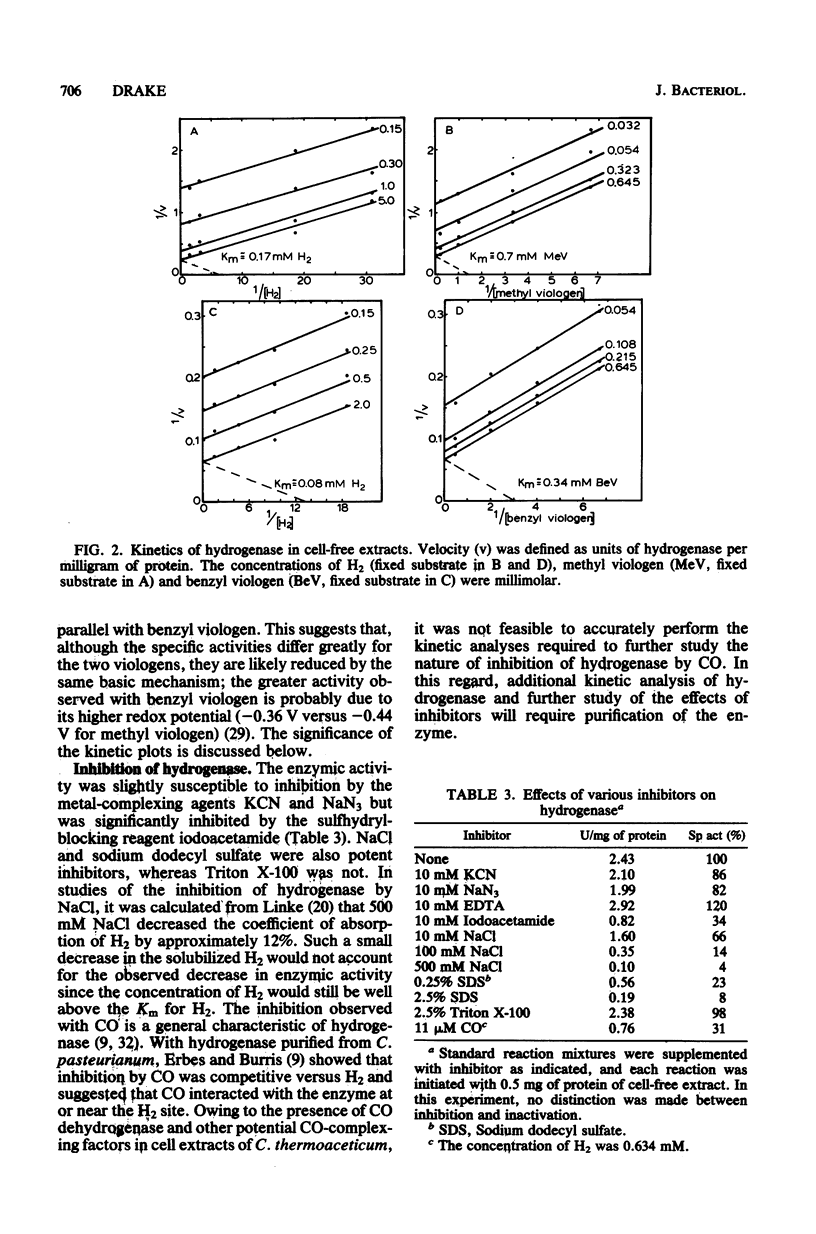
Cell-free extracts of the homoacetate-fermenting bacterium Clostridium thermoaceticum were shown to catalyze the hydrogen-dependent reduction of various artificial electron acceptors. The activity of the hydrogenase was optimal at pH 8.5 to 9 and was extremely sensitive to aeration. EDTA did not significantly reduce the liability of the enzymic activity to oxidation (aeration). At 50 degrees C, when both methyl viologen and hydrogen were at saturating concentrations with respect to hydrogenase, the specific activity of cell-free extracts approximated 4 mumol of H2 oxidized per min per mg of protein; fourfold higher specific activities were obtained when benzyl viologen was utilized as an electron acceptor. Activity stains of polyacrylamide gels demonstrated the presence of a single hydrogenase band, suggesting that the catalytic activity in cell extracts was due to a single enzyme. The activity was stable for at least 32 min at 55 degrees C but was slowly inactivated at 70 degrees C. NAD, NADP, flavin adenine dinucleotide, flavin mononucleotide, and ferredoxin were not significantly reduced, but possible reduction of the particulate b-type cytochrome of C. thermoaceticum was observed. NaCl, sodium dodecyl sulfate, iodoacetamide, and CO were shown to inhibit catalysis. A kinetic study is presented, and the possible physiologic roles for hydrogenase in C. thermoaceticum ar discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W., Mortenson L. E., Chen J. S. Hydrogenase. Biochim Biophys Acta. 1980 Dec;594(2-3):105–176. doi: 10.1016/0304-4173(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Andreesen J. R., Schaupp A., Neurauter C., Brown A., Ljungdahl L. G. Fermentation of glucose, fructose, and xylose by Clostridium thermoaceticum: effect of metals on growth yield, enzymes, and the synthesis of acetate from CO 2 . J Bacteriol. 1973 May;114(2):743–751. doi: 10.1128/jb.114.2.743-751.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- Drake H. L., Hu S. I., Wood H. G. Purification of carbon monoxide dehydrogenase, a nickel enzyme from Clostridium thermocaceticum. J Biol Chem. 1980 Aug 10;255(15):7174–7180. [PubMed] [Google Scholar]
- Drake H. L. Occurrence of nickel in carbon monoxide dehydrogenase from Clostridium pasteurianum and Clostridium thermoaceticum. J Bacteriol. 1982 Feb;149(2):561–566. doi: 10.1128/jb.149.2.561-566.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbes D. L., Burris R. H. The kinetics of methyl viologen oxidation and reduction by the hydrogenase from Clostridium pasteurianum. Biochim Biophys Acta. 1978 Jul 7;525(1):45–54. doi: 10.1016/0005-2744(78)90198-5. [DOI] [PubMed] [Google Scholar]
- Fontaine F. E., Peterson W. H., McCoy E., Johnson M. J., Ritter G. J. A New Type of Glucose Fermentation by Clostridium thermoaceticum. J Bacteriol. 1942 Jun;43(6):701–715. doi: 10.1128/jb.43.6.701-715.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Heine E., Finck A., Friedrich C. G. Nickel requirement for active hydrogenase formation in Alcaligenes eutrophus. J Bacteriol. 1981 Mar;145(3):1144–1149. doi: 10.1128/jb.145.3.1144-1149.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald M., Andreesen J. R., LeGall J., Ljungdahl L. G. Presence of cytochrome and menaquinone in Clostridium formicoaceticum and Clostridium thermoaceticum. J Bacteriol. 1975 Apr;122(1):325–328. doi: 10.1128/jb.122.1.325-328.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haschke R. H., Campbell L. L. Purification and properties of a hydrogenase from Desulfovibrio vulgaris. J Bacteriol. 1971 Jan;105(1):249–258. doi: 10.1128/jb.105.1.249-258.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. I., Drake H. L., Wood H. G. Synthesis of acetyl coenzyme A from carbon monoxide, methyltetrahydrofolate, and coenzyme A by enzymes from Clostridium thermoaceticum. J Bacteriol. 1982 Feb;149(2):440–448. doi: 10.1128/jb.149.2.440-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. M., Klibanov A. M., Kaplan N. O., Kamen M. D. The effect of electron carriers and other ligands on oxygen stability of clostridial hydrogenase. Biochim Biophys Acta. 1981 Jun 15;659(2):457–465. doi: 10.1016/0005-2744(81)90071-1. [DOI] [PubMed] [Google Scholar]
- Klibanov A. M., Kaplan N. O., Kamen M. D. A rationale for stabilization of oxygen-labile enzymes: application to a clostridial hydrogenase. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3640–3643. doi: 10.1073/pnas.75.8.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibanov A. M., Kaplan N. O., Kamen M. D. Chelating agents protect hydrogenase against oxygen inactivation. Biochim Biophys Acta. 1979 Aug 14;547(2):411–416. doi: 10.1016/0005-2728(79)90021-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lappi D. A., Stolzenbach F. E., Kaplan N. O., Kamen M. D. Immobilization of hydrogenase on glass beads. Biochem Biophys Res Commun. 1976 Apr 19;69(4):878–884. doi: 10.1016/0006-291x(76)90455-1. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G. Total synthesis of acetate from CO2 by heterotrophic bacteria. Annu Rev Microbiol. 1969;23:515–538. doi: 10.1146/annurev.mi.23.100169.002503. [DOI] [PubMed] [Google Scholar]
- Lovenberg W., Sobel B. E. Rubredoxin: a new electron transfer protein from Clostridium pasteurianum. Proc Natl Acad Sci U S A. 1965 Jul;54(1):193–199. doi: 10.1073/pnas.54.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar R. C., Sprott G. D. Solubilization and properties of a particulate hydrogenase from Methanobacterium strain G2R. J Bacteriol. 1979 Jul;139(1):231–238. doi: 10.1128/jb.139.1.231-238.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortenson L. E. Purification and properties of hydrogenase from Clostridium pasteurianum. Methods Enzymol. 1978;53:286–296. doi: 10.1016/s0076-6879(78)53035-8. [DOI] [PubMed] [Google Scholar]
- Schulman M., Ghambeer R. K., Ljungdahl L. G., Wood H. G. Total synthesis of acetate from CO2. VII. Evidence with Clostridium thermoaceticum that the carboxyl of acetate is derived from the carboxyl of pyruvate by transcarboxylation and not by fixation of CO2. J Biol Chem. 1973 Sep 25;248(18):6255–6261. [PubMed] [Google Scholar]
- Yagi T., Honya M., Tamiya N. Purification and properties of hydrogenases of different origins. Biochim Biophys Acta. 1968 Apr 2;153(3):699–705. doi: 10.1016/0005-2728(68)90197-7. [DOI] [PubMed] [Google Scholar]
- Yagi T., Kimura K., Daidoji H., Sakai F., Tamura S. Properties of purified hydrogenase from the particulate fraction of Desulfovibrio vulgaris, Miyazaki. J Biochem. 1976 Mar;79(3):661–671. doi: 10.1093/oxfordjournals.jbchem.a131111. [DOI] [PubMed] [Google Scholar]
- Yang S. S., Ljungdahl L. G., Dervartanian D. V., Watt G. D. Isolation and characterization of two rubredoxins from Clostridium thermoaceticum. Biochim Biophys Acta. 1980 Mar 7;590(1):24–33. doi: 10.1016/0005-2728(80)90143-7. [DOI] [PubMed] [Google Scholar]




