Abstract
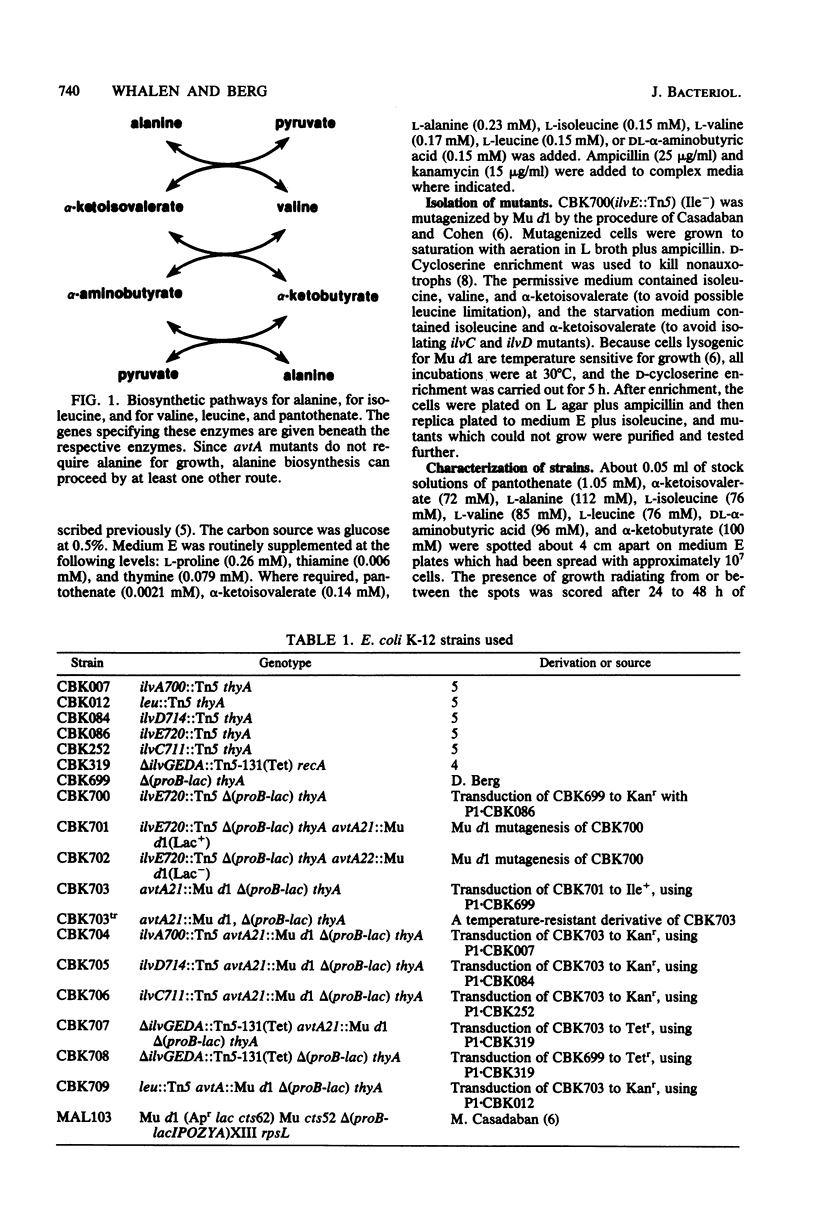
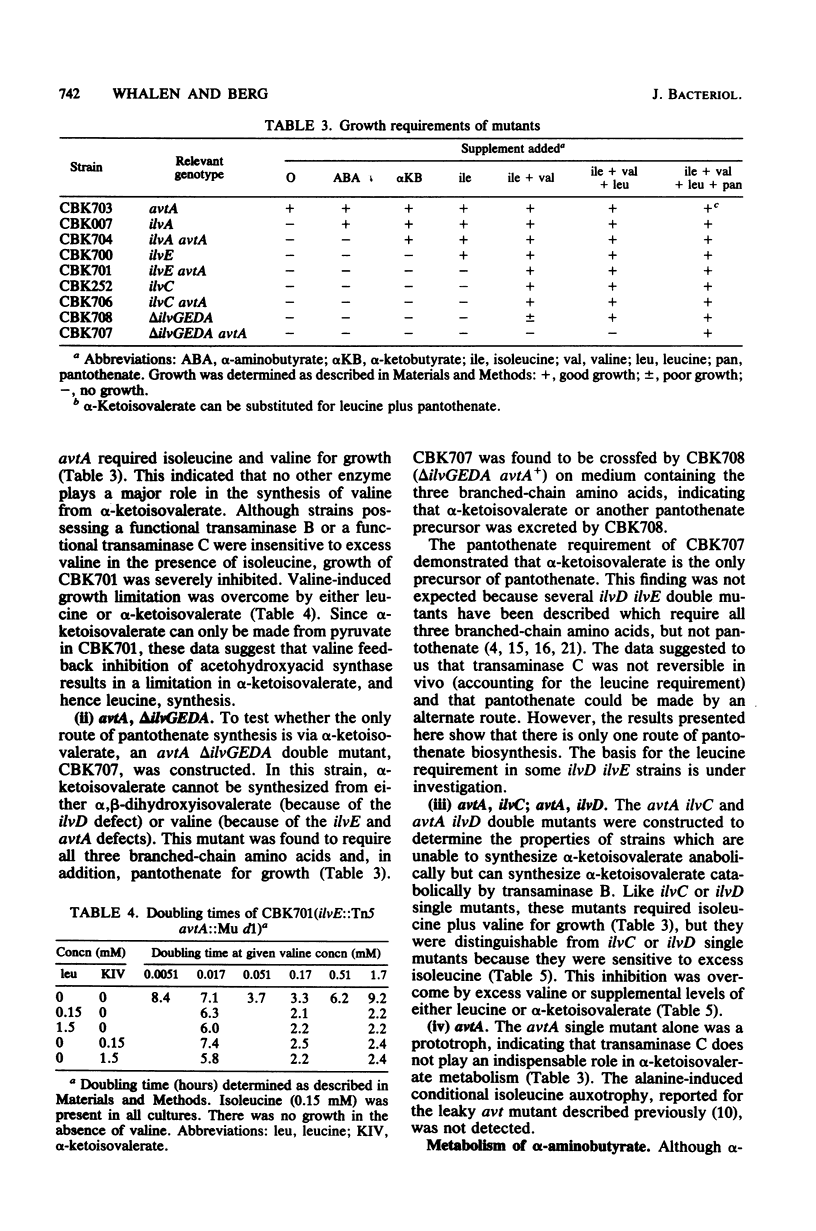
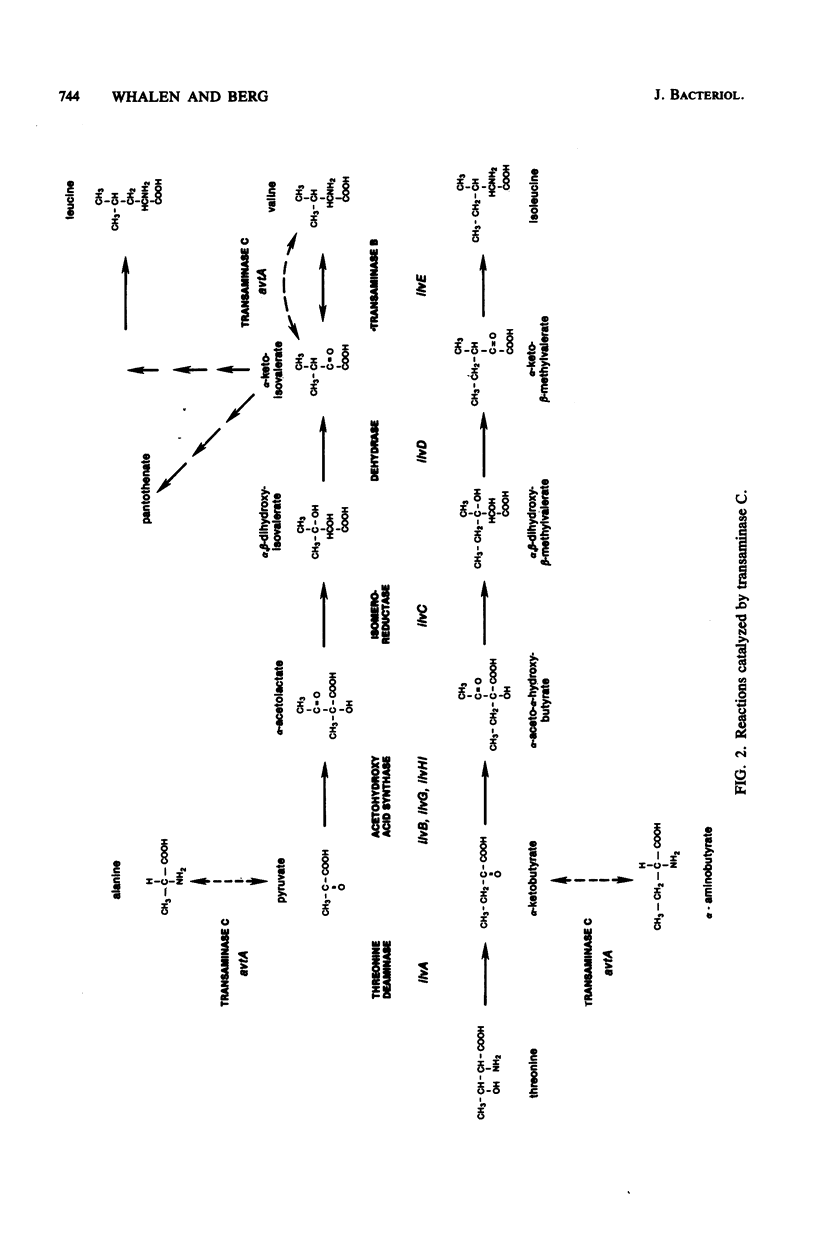
Escherichia coli can synthesize alpha-ketoisovalerate, the precursor of valine, leucine, and pantothenate, by three routes: anabolically via dihydroxyacid dehydrase and catabolically via both the branched-chain amino acid transaminase (transaminase B) and the alanine-valine transaminase (transaminase C). An E. coli K-12 mutant devoid of transaminase C (avtA) was isolated by mutagenizing an isoleucine-requiring strain devoid of transaminase B (ilvE::Tn5) with Mu d1(Ap lac) and selecting for valine-requiring derivatives which were ampicillin resistant, Lac+, able to crossfeed an ilvD mutant, and unable to grow on alpha-ketoisovalerate in place of valine. Strains defective in one, two, or all three alpha-ketoisovalerate metabolic enzymes were constructed, and their properties were analyzed. The data indicated that avtA is the structural gene for transaminase C, that transaminase C is a single enzyme species, and that the sole pathway for pantothenate biosynthesis is from alpha-ketoisovalerate. The data further showed that isoelectric inhibits the transaminase B-catalyzed deamination of valine in vivo.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. V. alpha-Ketoisovaleric acid accumulation. J Biol Chem. 1953 Nov;205(1):475–482. [PubMed] [Google Scholar]
- Adams C. W., Lawther R. P., Hatfield G. W. The ilvEDA operon of Escherichia coli K12 encodes only one valine-alpha-ketoglutarate transaminase activity. Biochem Biophys Res Commun. 1979 Jul 27;89(2):650–658. doi: 10.1016/0006-291x(79)90679-x. [DOI] [PubMed] [Google Scholar]
- Berg C. M., Shaw K. J., Berg D. E. The ilvG gene is expressed in Escherichia coli K-12. Gene. 1980 Dec;12(1-2):165–170. doi: 10.1016/0378-1119(80)90028-1. [DOI] [PubMed] [Google Scholar]
- Berg C. M., Shaw K. J. Organization and regulation of the ilvGEDA operon in Salmonella typhimurium LT2. J Bacteriol. 1981 Feb;145(2):984–989. doi: 10.1128/jb.145.2.984-989.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg C. M., Shaw K. J., Vender J., Borucka-Mankiewicz M. Physiological characterization of polar Tn5-induced isoleucine-valine auxotrophs in Escherichia coli K.12: evidence for an internal promoter in the ilvOGEDA operon. Genetics. 1979 Oct;93(2):308–319. [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. Use of 3H and 14C double-labeled glucose to assess in vivo pathways of amino acid biosynthesis in Escherichia coli. J Biol Chem. 1977 May 25;252(10):3392–3398. [PubMed] [Google Scholar]
- Curtiss R., 3rd, Charamella L. J., Berg C. M., Harris P. E. Kinetic and genetic analyses of D-cycloserine inhibition and resistance in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1238–1250. doi: 10.1128/jb.90.5.1238-1250.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J., Danchin A. Involvement of cyclic AMP and its receptor protein in the sensitivity of Escherichia coli K 12 toward serine: excretion of 2-ketobutyrate, a precursor of isoleucine. Mol Gen Genet. 1979 Nov;176(3):343–350. doi: 10.1007/BF00333096. [DOI] [PubMed] [Google Scholar]
- Gelfand D. H., Steinberg R. A. Escherichia coli mutants deficient in the aspartate and aromatic amino acid aminotransferases. J Bacteriol. 1977 Apr;130(1):429–440. doi: 10.1128/jb.130.1.429-440.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. L. Cysteine and growth inhibition of Escherichia coli: threonine deaminase as the target enzyme. J Bacteriol. 1981 Feb;145(2):1031–1035. doi: 10.1128/jb.145.2.1031-1035.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Calhoun D. H. Intracellular roles of microbial aminotransferases: overlap enzymes across different biochemical pathways. Crit Rev Microbiol. 1981;8(3):229–266. doi: 10.3109/10408418109085080. [DOI] [PubMed] [Google Scholar]
- Kline E. L., Manross D. N., Jr, Warwick M. L. Multivalent regulation of isoleucine-valine transaminase in an Escherichia coli K-12 ilvA deletion strain. J Bacteriol. 1977 May;130(2):951–953. doi: 10.1128/jb.130.2.951-953.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEAVITT R. I., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. XI. Valine inhibition of the growth of Escherichia coli strain K-12. J Bacteriol. 1962 Mar;83:624–630. doi: 10.1128/jb.83.3.624-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Peng F. C., Hermodson M. A., Kohlhaw G. B. Transaminase B from Escherichia coli: quaternary structure, amino-terminal sequence, substrate specificity, and absence of a separate valine-alpha-ketoglutarate activity. J Bacteriol. 1979 Aug;139(2):339–345. doi: 10.1128/jb.139.2.339-345.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGilvray D., Umbarger H. E. Regulation of transaminase C synthesis in Escherichia coli: conditional leucine auxotrophy. J Bacteriol. 1974 Nov;120(2):715–723. doi: 10.1128/jb.120.2.715-723.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. II. IDENTIFICATION OF TWO OPERATOR GENES. J Bacteriol. 1965 Mar;89:654–660. doi: 10.1128/jb.89.3.654-660.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDMAN D., MEISTER A. Transamination in Escherichia coli. J Biol Chem. 1953 Feb;200(2):591–604. [PubMed] [Google Scholar]
- Raskó I., Alföldi L. Biosynthetic L-threonine deaminase as the origin of L-serine sensitivity of Escherichia coli. Eur J Biochem. 1971 Aug 16;21(3):424–427. doi: 10.1111/j.1432-1033.1971.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Shaw K. J., Berg C. M. Radioenzymatic assay for the acetohydroxy acid synthase-catalyzed synthesis of alpha-aceto-alpha-hydroxybutyrate. Anal Biochem. 1980 Jun;105(1):101–105. doi: 10.1016/0003-2697(80)90429-7. [DOI] [PubMed] [Google Scholar]
- Smith J. M., Smolin D. E., Umbarger H. E. Polarity and the regulation of the ilv gene cluster in Escherichia coli strain K-12. Mol Gen Genet. 1976 Oct 18;148(2):111–124. doi: 10.1007/BF00268374. [DOI] [PubMed] [Google Scholar]
- UMBARGER H. E., MUELLER J. H. Isoleucine and valine metabolism of Escherichia coli. I. Growth studies on amino acid-deficient mutants. J Biol Chem. 1951 Mar;189(1):277–285. [PubMed] [Google Scholar]



