Abstract
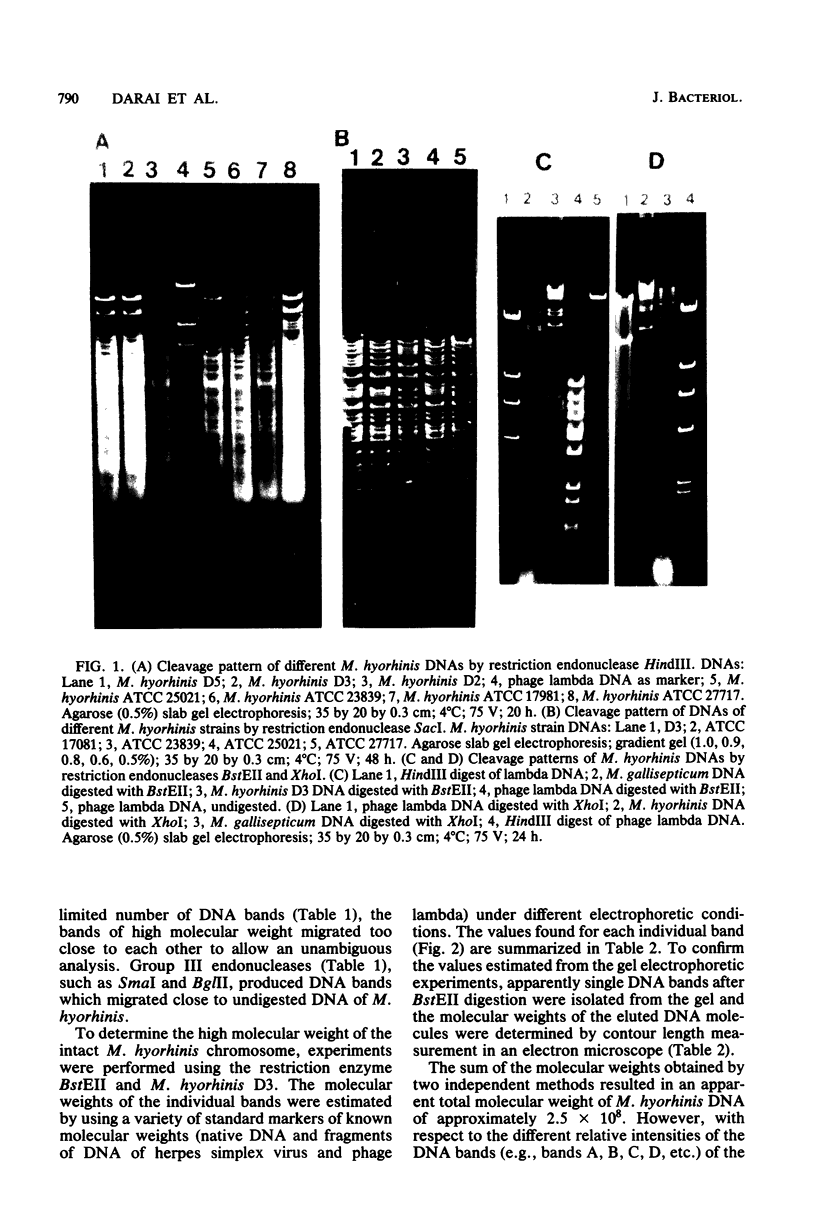
The chromosome of Mycoplasma hyorhinis was analyzed by using different restriction endonucleases and electron microscopy. It was found that restriction enzymes BstEII, XhoI, and SacI are the enzymes of choice for analysis and characterization of M. hyorhinis. The bands resulting from digestion of M. hyorhinis DNA with BstEII had apparent molecular weights ranging from 1.2 X 10(6) to 75 X 10(6). The apparent total molecular weight of DNA was calculated from the molecular weights of the individual bands and found to be 251 X 10(6). Electron microscopic contour length measurements of the largest DNA fragments verified the molecular weight values calculated from gel analysis. Electron microscopic contour length measurements of intact DNA of M. hyorhinis revealed a molecular weight of 5.4 +/- 5 X 10(8). The discrepancy between the values of molecular weight of M. hyorhinis DNA as determined by restriction enzyme analysis and contour length measurement is based on the fact that some of the DNA fragments which migrate as an apparent single band in the agarose gel really are double or multiple DNA fragments.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Darai G., Flügel R. M., Zöller L., Matz B., Kreig A., Gelderblom H., Delius H., Leach R. H. The plaque-forming factor for mink lung cells present in cytomegalovirus and herpes-zoster virus stocks identified as Mycoplasma hyorhinis. J Gen Virol. 1981 Jul;55(Pt 1):201–205. doi: 10.1099/0022-1317-55-1-201. [DOI] [PubMed] [Google Scholar]
- Fangman W. L. Separation of very large DNA molecules by gel electrophoresis. Nucleic Acids Res. 1978 Mar;5(3):653–665. doi: 10.1093/nar/5.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin R. F., Pomeroy A. P., Whittlestone P. Characterization of Mycoplasma suipneumonia: a mycoplasma causing enzootic pneumonia of pigs. J Hyg (Lond) 1967 Mar;65(1):85–96. doi: 10.1017/s0022172400045563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Müller W., Hattesohl I., Schuetz H. J., Meyer G. Polyethylene glycol derivatives of base and sequence specific DNA ligands: DNA interaction and application for base specific separation of DNA fragments by gel electrophoresis. Nucleic Acids Res. 1981 Jan 10;9(1):95–119. doi: 10.1093/nar/9.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Razin S. Methylated bases in mycoplasmal DNA. Nucleic Acids Res. 1980 Mar 25;8(6):1383–1390. doi: 10.1093/nar/8.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teplitz M. Isolation of folded chromosomes from Mycoplasma hyorhinis. Nucleic Acids Res. 1977;4(5):1505–1512. doi: 10.1093/nar/4.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]