Abstract
We have developed an approach based on a differential mass spectrometric analysis to detect molecules induced during the immune response of Drosophila, regardless of their biological activities. For this, we have applied directly matrix-assisted laser desorption/ionization MS to hemolymph samples from individual flies before and after an immune challenge. This method provided precise information on the molecular masses of immune-induced molecules and allowed the detection, in the molecular range of 1.5–11 kDa, of 24 Drosophila immune-induced molecules (DIMs). These molecules are all peptides, and four correspond to already characterized antimicrobial peptides. We have further analyzed the induction of the various peptides by immune challenge in wild-type flies and in mutants with a compromised antimicrobial response. We also describe a methodology combining matrix-assisted laser desorption ionization time-of-flight MS, HPLC, and Edman degradation, which yielded the peptide sequence of three of the DIMs. Finally, molecular cloning and Northern blot analyses revealed that one of the DIMs is produced as a prepropeptide and is inducible on a bacterial challenge.
Drosophila, similar to other insects, has the capacity to mount an efficient host defense against microorganisms that induce both cellular and humoral reactions (for review, see ref. 1). The cellular response is marked by phagocytosis and capsule formation. The humoral immunity consists of proteolytic cascades in the hemolymph, which lead to localized melanization and coagulation, and of the induction of antimicrobial peptides that are synthesized by the fat body and secreted into the body fluid (1). How a starting infection is signaled to the fat body is not fully understood. It has been proposed that proteolytic cascades are triggered by cell wall components of the microorganisms, leading to the cleavage of circulating cytokine-like proteins into active ligands that interact with transmembrane receptors of fat body cells (2). Other signaling mechanisms leading from the site of infection to the fat body have been proposed, but to date no molecule has been identified in the hemolymph of infected larvae or adults of Drosophila that could be related clearly to infection, with the exception of the antimicrobial peptides. In the latter case, it must be stressed that these molecules were either extracted from tens of thousands of immune-challenged whole flies and detected by their antimicrobial activity, or their presence was inferred by molecular cloning techniques based on homologies with peptides isolated from larger insects (for review, see ref. 1).
To detect molecules involved in the immune response of Drosophila regardless of their biological activities, we developed an approach based on a differential analysis by MS of the components present in the hemolymph of single control and bacteria-challenged flies. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) MS is the most appropriate MS technique for this kind of study, since it usually produces monoprotonated molecular ions leading to spectra without multiple charging or significant fragmentations (3). MALDI-TOF MS allows the easy and rapid detection of molecules at subfemtomolar to attomolar concentrations with an accuracy up to one part per million (4). This MS method is also more tolerant to impurities such as buffers, salts, additives, and detergents than are the other MS techniques (5). Altogether, these characteristics make MALDI-TOF MS very suitable for the direct analysis of biological tissues or fluids (6, 7).
The MALDI-TOF MS differential display methodology that we have applied in this study on individual immune-challenged vs. nonchallenged flies has provided, in a first step, the molecular masses of induced substances. Subsequently, we have used this information on the molecular masses of the induced molecules to screen HPLC fractions from hemolymph batches (pools of 20 individuals) by MALDI-TOF MS to isolate sufficient material for structural characterization. We demonstrate the validity of this approach and report the detection of a surprisingly high number of peptides induced by an experimental challenge. Some of these peptides correspond to already characterized antimicrobial peptides, whereas the majority appear to be novel. We also illustrate the validity of this methodology by structurally characterizing three of the induced peptides from a very low number (140) of challenged flies. Molecular cloning experiments based on one of these molecules indicate that the molecule is induced by bacterial challenge, thus confirming its involvement in the immune response of Drosophila.
MATERIALS AND METHODS
Drosophila Strains, Immunization, and Hemolymph Collection.
Oregon R flies were used as a standard wild-type strain. imd is a line homozygous for the imd mutation, which alters the expression of the genes encoding Drosophila antibacterial peptides (8). Toll10b is a dominant gain-of-function allele of Toll that induces a constitutive expression of the antifungal peptide drosomycin (2). Drosophila flies with a Tollr632/Tolll-RXA genotype exhibit a strong Toll− phenotype that significantly reduces the inducibility of drosomycin (2).
Adult flies were individually pricked under CO2 with a needle dipped into a combined bacterial pellet of Escherichia coli 1106 and Micrococcus luteus obtained after centrifugation of 37°C overnight cultures in Luria–Bertani medium. For each strain, unchallenged adults (naive Drosophila) were kept as controls.
Hemolymph of Drosophila adults was collected with the help of a nanoinjector (Nanoject, Drummond Scientific, Broomall, PA) used as a glass capillary holder. Hemolymph was collected by capillarity, retrieved by mechanical pressure ejection, and was either directly deposited on the mass sample target for MS analysis or diluted with 0.1% trifluoroacetic acid (TFA) for HPLC analysis.
MALDI-TOF MS Analysis.
Hemolymph samples were prepared as previously described (6): 0.5 μl of a 1:1 mixture of α-cyano-4-hydroxycinnamic acid (4HCCA, 40 mg/ml in acetone) and nitrocellulose (NC, 40 mg/ml in acetone) diluted 1:1 with isopropyl alcohol was deposited on the probe tip. The hemolymph samples were directly loaded onto this NC/4HCCA bed and were covered by 0.5 μl of a second matrix solution, which consisted of 4HCCA at 7 mg/ml in 0.1% TFA/acetonitrile (1:1, vol/vol). After air drying, this preparation was rinsed with 1 μl of 0.1% TFA, which was flushed away after a few seconds (9). For HPLC analysis, 0.5 μl of the fractions was deposited on a thin layer of 4HCCA crystals made by fast evaporation of 0.5 μl of a saturated solution in acetone (10). After solvent evaporation under mild vacuum, samples were washed with 1 μl of 0.1% TFA.
MALDI mass spectra were acquired on a Bruker (Bremen, Germany) BIFLEX MALDI-TOF mass spectrometer. This instrument has a maximum acceleration potential of 30 kV and may be operated in either linear or reflector mode. Ionization was accomplished with the 337-nm beam from a nitrogen laser with a repetition rate of 3 Hz. A camera mounted on a microscope allowed the inspection of the sample crystallization homogeneity before measurement.
The MALDI mass spectra of hemolymph from naive Drosophila were externally calibrated with a mixture of standard peptides [angiotensin II, corticotropin-(18–39), and bovine insulin with [M+H]+ at m/z 1,047.2, 2,466.1, and 5,734.6, respectively]. The MALDI mass spectra of hemolymph from immune-challenged insects were first externally calibrated with the same standard peptides. To obtain a more accurate mass assignment of the peptides, an internal calibration was achieved subsequently by using the natural identified antimicrobial peptides (monoglycosylated drosocin, metchnikowin, and drosomycin with respective [M+H]+ at m/z 2,402.9, 3,046.4, and 4,890.5). For the mass analysis of the HPLC fractions, an external calibration was performed with recombinant drosomycin, synthetic nonglycosylated drosocin ([M+H]+ at m/z 2,199.5), and synthetic metchnikowin.
Analysis by HPLC of Drosophila Hemolymph.
Hemolymph was collected from two distinct batches of 20 control and 20 bacteria-challenged flies (24 h post-challenge). The samples were subjected separately to RP-HPLC on an Aquapore RP 300 C8 column (1 × 100 mm, Brownlee Lab) protected by a guard filter (Ultra-Low Dead-Volume Precolumn Filter, Upchurch Scientific, Oak Harbor, WA) and were equilibrated in acidified water (0.05% TFA). Separation of the hemolymph components was performed with a linear gradient of 0–80% acetonitrile in 0.05% TFA over 80 min at a flow rate of 80 μl/min. Fractions were hand-collected in low protein-binding Eppendorf tubes, dried under vacuum, and reconstituted in MilliQ water (Millipore) before being subjected to MALDI-TOF measurements. Final purifications of peptides were performed in appropriate linear biphasic gradients. HPLC purifications were carried out under controlled temperature at 35°C on a Waters HPLC system (Waters 626 pump) attached to a tunable-absorbance detector (Waters 486). Column effluent was monitored by its UV absorption at 214 nm.
Microsequence Analysis.
Peptides were subjected to Edman degradation on a pulse liquid automatic sequenator (Applied Biosystems, model 473A).
RESULTS
A Differential Analysis by MALDI-TOF MS of Molecules Present in the Hemolymph of Immune-Challenged and Naive Flies.
We have collected the hemolymph (0.1 μl) from a single adult fly 24 h after a bacterial challenge, and have subjected the sample to MALDI-TOF MS in the linear mode. The mass spectrum obtained for m/z values between 1,500 and 11,000 is presented in Fig. 1 and is compared with that of a control fly. This experiment was repeated on individuals from both sexes up to 50 times, yielding highly reproducible results. However, the mass signals for the compounds with higher masses exhibited poor intensities and the data were less reproducible. In the present study, we have therefore restricted our analysis to compounds with masses below 11 kDa. The discrepancy in resolution and reproducibility of the mass signals for compounds with masses below and over 11 kDa is probably related to the observation that the sample preparation as performed here (NC and 4HCCA) favors the ionization of compounds with a mass below 10 kDa (6). We are exploring the possibilities of improving the intensities of the mass signals of higher mass compounds by varying the matrix preparations (e.g., dried-droplet and thin-layer methods with 4HCCA or sinapinic acid), and by introducing pretreatments of the biological samples for enrichment of high molecular mass compounds.
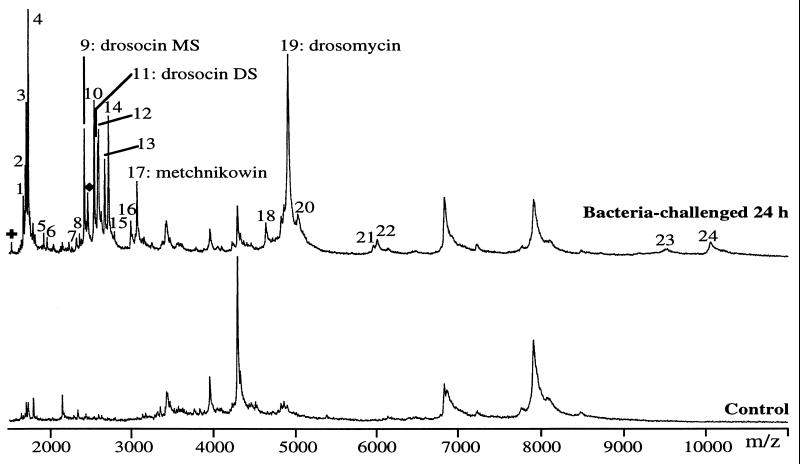
Figure 1.
Differential MALDI-TOF MS analysis of hemolymph from a control and a 24-h bacteria-challenged adult Drosophila. The hemolymph was collected from a single fly (0.1 μl) and analyzed by MALDI-TOF MS in the positive linear mode by using NC and 4HCCA in the sample preparation (see Materials and Methods). The singly charged ions of the molecules induced after bacterial challenge are numbered from 1 to 24. Among these, four peaks have masses corresponding to already characterized antimicrobial peptides, which are designated by their names. MS and DS refer to the monosaccharide and the disaccharide glycoforms of drosocin, respectively. ✚ and ♦ are the doubly charged ions of metchnikowin and drosomycin, respectively.
The mass spectrum obtained with hemolymph from an immune-challenged fly is remarkably more complex than that from a control fly (Fig. 1), because it contains, in addition to the peaks present in the control sample, 24 distinct peaks (numbered from 1 to 24). In the experiments described above, immune challenge had been performed by pricking the fly with a needle dipped into bacterial cultures. We were wondering whether some of the 24 inducible peaks could result from the introduction of bacterial constituents during this procedure and/or following bacterial lysis. Therefore, we repeated the pricking experiments with sterile needles devoid of bacteria. The spectra obtained under these conditions were similar to those from bacteria-challenged flies (data not shown), eliminating the possibility of a contamination of the spectra by bacterial products (see also Discussion).
With an external calibration and at their ionization threshold, where the best resolution and mass accuracies are obtained (11), peaks 9, 11, 17, and 19 were detected at m/z 2,402.9, 2,566.1, 3,046.6, and 4,890.6, respectively. The masses of these peaks correspond to known antimicrobial peptides produced by the fat body after immune challenge and secreted into the hemolymph (1): (i) peaks 9 and 11 at m/z 2,402.9 and 2,566.1 correspond to the anti-Gram-negative O-glycopeptide drosocin (12, 13) carrying an N-acetylgalactosamine monosaccharide (drosocin MS) and an N-acetylgalactosamine-galactose disaccharide (drosocin DS), respectively, on the threonine residue (calculated masses of 2,401.9 and 2,564.4 Da, respectively); (ii) peak 17 at m/z 3,046.6 corresponds to metchnikowin (calculated mass of 3,045.4 Da), which exhibits anti-Gram-positive and antifungal activities (14); (iii) peak 19 at m/z 4,890.6 corresponds to the antifungal peptide drosomycin (15) (calculated mass of 4,889.5 Da). We were unable to detect other established antimicrobial peptides of Drosophila. In particular, we did not observe the defensin mass signal at 4,354 Da (16), possibly as a result of the extremely low concentration of this peptide in the hemolymph of bacteria-challenged flies [<2 μM, as compared with 100 μM for drosomycin, 40 μM for the two glycoforms of drosocin, and 10 μM for metchnikowin (P.B., unpublished work)]. This interpretation was corroborated by experiments in which we added 5 ng of purified Drosophila defensin to the hemolymph of a bacteria-challenged fly (leading to an estimated final concentration of 10 μM) before mass analysis; a mass signal at m/z 4,356 became apparent under these conditions (data not shown). We also failed to detect cecropin A (calculated mass of 4,156 Da), although its concentration is around 20 μM in the hemolymph of immune flies (P.B., unpublished work). We believe that the response of cecropin is minimized or even suppressed during MALDI-TOF measurements of our samples by the vicinity of the high peak of drosomycin at m/z 4,890.6. To examine this hypothesis, we have tested the preferential ionization of drosomycin by adding synthetic cecropin A in the presence or absence of recombinant drosomycin to hemolymph samples collected from naive flies. The concentration of the added peptides mimicked those found in bacteria-challenged flies. When cecropin A alone was added, a clearcut peak was observed with the expected m/z of 4,157. However, in the presence of drosomycin, the ionization of cecropin A was clearly suppressed (data not shown).
For 20 of the 24 peaks induced by immune challenge and collectively referred to as Drosophila immune-induced molecules (DIM) hereafter, no identity could be proposed.
Additionally, we have assigned more accurate m/z to the unknown DIMs with masses below 5 kDa by measuring them in the reflector mode with the detected and already known antimicrobial peptides as internal calibrants. The following average m/z of the molecular ions of DIMs 1–19 were: 1, 1,667.6; 2, 1,690.0; 3, 1,701.7; 4, 1,723.0; 5, 1,915.0; 6, 1,956.2; 7, 2,308.1; 8, 2,349.2; 9, 2,402.9 (drosocin MS); 10, 2,521.6; 11, 2,565.0 (drosocin DS); 12, 2,574.0; 13, 2,652.0; 14, 2,695.0; 15, 2,768.6; 16, 2,972.1; 17, 3,046.6 (metchnikowin); 18, 4,626.0, and 19, 4,890.8 (drosomycin). For DIMs 20–24 with a mass above 5 kDa, the measured m/z values were obtained in the linear mode with the identified antimicrobial peptides as internal calibrants: 20, 5,023; 21, 5,939; 22, 5,984; 23, 9,521, and 24, 10,064.
We have scanned the Swiss-Prot and Protein Information Resource databanks, but have been unable to assign known Drosophila hemolymph proteins to any of the recorded masses of the DIMs, except for the antimicrobial peptides discussed above. We have also made sure that no DIMs corresponded to potential degradation products of these antimicrobial peptides.
Kinetics of Appearance of the DIMs in the Hemolymph.
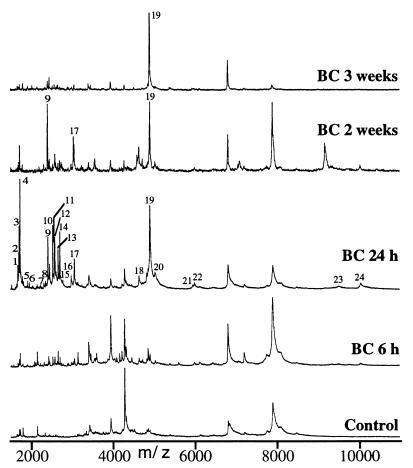
Next, we collected, for MS analysis, hemolymph from individual immune-induced flies at various time intervals after a bacterial challenge (30 min, 1 h, every 2 h up to one day, and thereafter every day up to 3 weeks). More than 100 spectra were recorded, of which several significant results are presented in Fig. 2. The analysis of these spectra led to the following observations: (i) as early as 6 h after challenge, most of the 24 DIMs were detectable; this was the case in particular for the four above-mentioned antimicrobial peptides; (ii) the intensities of the various peaks increased markedly between 6 h and 24 h to maximum values; (iii) over the next 3 weeks, the intensities of the peaks decreased gradually, with some variations between individual flies. Drosomycin remained detectable up to 3 weeks after challenge in most flies and the disaccharide form of drosocin had disappeared after 2 weeks, whereas the monosaccharide form persisted for up to 3 weeks. Metchnikowin was not detectable 3 weeks after challenge. The 20 unknown DIMs became undetectable between 2 and 3 weeks after the beginning of the experiment.
Figure 2.
Kinetics of appearance of DIMs in the hemolymph of bacteria-challenged and control flies. MALDI-TOF mass spectra of hemolymph from a control fly and from flies 6 h, 24 h, 2 and 3 weeks after bacterial challenge (BC) were obtained under the conditions described in Materials and Methods. The singly charged ions of the DIMs are numbered from 1 to 24 as in Fig. 1.
Induction of the DIMs in Mutants with Alterations in Their Immune Response.
Recent studies based on a genetic analysis have pointed to the existence of two pathways controlling the expression of the antimicrobial peptide genes in adult Drosophila (2, 8). The Toll pathway, which comprises the intracellular components of the dorsoventral signaling pathway (except for dorsal) and the extracellular Toll ligand spaetzle, controls the expression of the antifungal peptide drosomycin. Antibacterial genes are induced either by a distinct pathway involving the immune deficiency gene (imd) or by combined activation of both the imd and Toll pathways. The studies that unravelled the existence of these pathways were essentially based on the analysis of transcriptional profiles of the antimicrobial peptide genes in various mutant backgrounds. The methodology that we are introducing here allows the direct recording of the peptides in the hemolymph of individual flies. Consequently, we have subjected to MALDI-TOF MS analysis hemolymph samples collected from mutant flies. The results, which are illustrated in Fig. 3, lead to the following conclusions.
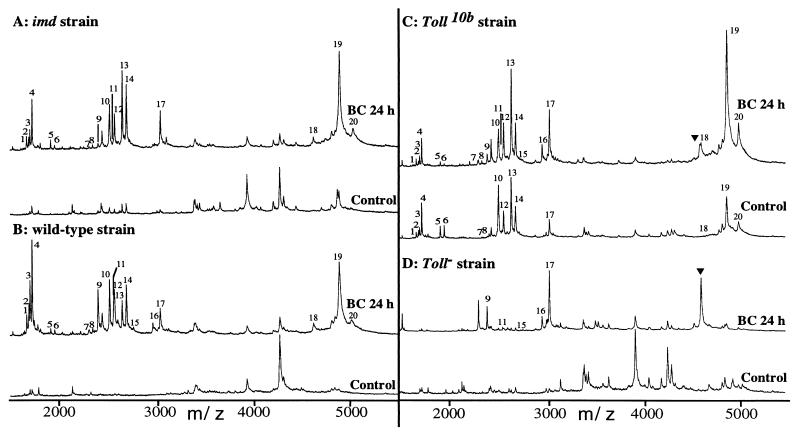
Figure 3.
Induction of DIMs in mutants with alterations in their immune response. The MALDI-TOF mass spectra of hemolymph from control and 24-h bacteria-challenged (BC 24 h) Drosophila of the imd (A), wild-type (B), Toll10b (C) and Toll− (D) strains were obtained in the linear mode by using NC and 4HCCA in the sample preparation, as reported in Materials and Methods. The DIMs numbered from 1 to 20 are reported and ▾ represents a molecule at m/z 4,620 induced after a bacterial challenge in the hemolymph of Toll10b and Toll− mutant flies.
imd mutant background. When the mass spectra of hemolymph from bacteria-challenged imd flies (Fig. 3A) are compared with those of wild-type flies (Fig. 3B), similar signals are observed for 20 of the 24 peaks recorded (peaks 21–24 are not shown in the figure, but were identical for both types of flies). Only peaks 9 and 11, corresponding to the monosaccharide and the disaccharide glycoforms of drosocin, respectively, were considerably lower in imd mutants and peaks 15 and 16 were undetectable in this background. The strong induction of drosomycin by immune challenge was not affected in imd mutants, nor was that of metchnikowin.
Mutations in the Toll pathway.
In strains carrying a loss-of-function mutation for Toll (Toll−), only the peaks corresponding to drosocin and metchnikowin and peaks 15 and 16 were clearly induced after bacterial challenge (Fig. 3D). In unchallenged Toll gain-of-function mutants (Toll10b), all peaks were constitutively present (including peaks 21–24, not shown in the figure), with the exception of drosocin and peaks 15 and 16 (Fig. 3C). In particular, drosomycin, as well as metchnikowin, were present in the hemolymph. When these mutant flies were immune-challenged, drosocin (peaks 9 and 11) and peaks 15 and 16 were induced and the peaks corresponding to metchnikowin and drosomycin were stronger than in unchallenged Toll gain-of-function mutants. A molecule at m/z 4620 (symbolized by ▾ in Fig. 3 C and D) was induced by bacterial challenge in both types of Toll mutants; we did not detect this molecule in challenged wild-type or imd flies.
Structural Characterization of Three Novel Immune-Induced Molecules from Drosophila.
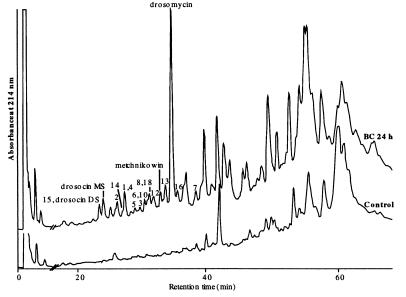
In a pilot experiment, we then attempted to fully characterize some of the unknown DIMs. For this, we collected hemolymph from 20 bacteria-challenged and 20 control flies in acidified water. The samples were subjected to RP-HPLC separation with a linear gradient of 0–80% acetonitrile in 0.05% TFA over 80 min (Fig. 4). We screened the various HPLC fractions by MALDI-TOF MS to select those containing the various DIMs. We observed that DIMs 1–19 with masses below 5 kDa were all present in the more hydrophilic fractions. During this procedure, we systematically compared the samples from challenged flies with extracts from control flies to ascertain that the molecules that we purified were indeed induced by immune challenge. Importantly, a Pronase treatment of the hemolymph from bacteria-challenged flies totally removed the DIMs, indicating their peptidic nature.
Figure 4.
RP-HPLC of acidic extracts obtained from the hemolymph of control and 24-h bacteria-challenged Drosophila. Hemolymph of 20 control and 20 bacteria-challenged flies (BC 24 h) were diluted in 0.1% TFA and were analyzed separately on an Aquapore RP300 C8 column (see Materials and Methods). Absorbance was monitored at 214 nm. The DIMs numbered from 1 to 19, revealed by differential MS analysis in Fig. 1, were detected by MALDI-TOF MS in the HPLC fractions of the hemolymph from bacteria-challenged flies. Four of the already characterized Drosophila antimicrobial peptides [drosocin monosaccharide (MS) and disaccharide (DS), metchnikowin, and drosomycin] are reported on the figure according to their names.
Second reversed-phase chromatographies of the fractions containing DIMs 1, 2, and 4 obtained after a first purification on hemolymph collected from 140 flies were analyzed in the same conditions as above, but with a shallower gradient of acetonitrile. This procedure yielded pure material of DIMs 1, 2, and 4, as confirmed by MALDI-TOF MS measurements, in sufficient quantities for Edman degradation. For DIM 4, the following sequence was obtained: GTVLIQTDNTQYIRT. The monoisotopic mass calculated from this sequence (1,721.9 Da) is higher by 1 Da than that of the monoisotopic mass measured by MALDI-TOF MS (1,720.9 Da). This difference can be explained by a carboxyl-terminal (C-terminal) amidation of the peptide. The peptide sequences obtained for DIM 1, GNVIINGDCRVCNVHG, and DIM 2, GNVVINGDCKYCNVHG, revealed that these peptides are isoforms. The difference of 3 Da obtained between the calculated (1,690.7 Da) and the measured (1,687.7 Da) monoisotopic molecular masses for DIMs 1 and 2 suggests that both peptides are C-terminally amidated and that both contain an intramolecular disulfide bridge. From the peptide sequence established for DIM 2, we designed degenerate oligonucleotide primer pools to perform 3′ and 5′ rapid amplification of cDNA ends PCR with RNA prepared from immune-challenged adults. The combined information from the 3′ and 5′ rapid amplification of cDNA ends fragments yielded a 360-bp sequence that contained an ORF of 46 codons, starting with an M codon and ending with a stop codon:1 10 20 30 40MKFFSVVTVFVLRSAGSGQTAVPLSPDPGNVVINGDCKYCNVHGGK
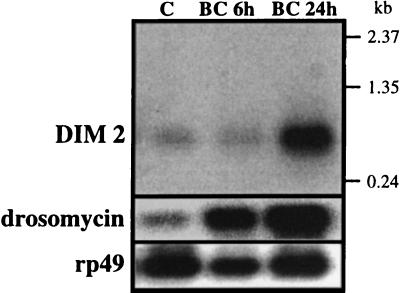
The 16 residues underlined in this sequence (G-29–G-44) match perfectly the sequence of DIM 2. In the deduced sequences, the DIM 2 peptide is preceded N-terminally by a putative hydrophobic signal peptide (residues M-1–A-21, in italics). Assuming that residue A-21 could correspond to a cleavage site for a signal peptidase, additional maturation steps by dipeptidyl aminopeptidases would be required to yield the sequence of DIM 2. Maturation of propeptides by removal of P-containing dipeptidyl groups has been reported for other immune-induced peptides in insects (17). The deduced amino-acid sequence shows G-45 and K-46 residues that were not found by Edman degradation. Assuming that the K-46 residue is cleaved by a carboxypeptidase, G-45 could serve for the C-terminal amidation of DIM 2, which is in agreement with the MALDI-TOF MS data. Finally, a Northern blot analysis performed with a radioactive cDNA probe showed that the messenger encoding the DIM 2 sequence has a size of approximately 480 bp. The level of preproDIM mRNA is low in normal adults, but noticeably increases 24 h after immune challenge, as is the case for the mRNAs encoding other immune-induced peptides, e.g., drosomycin (Fig. 5).
Figure 5.
Northern blot analysis of DIM 2 and drosomycin. 5 μg of poly(A) RNA from control (C) and bacteria-challenged (BC) adults were fractionated by denaturing electrophoresis in 1% agarose-formaldehyde gel with Mops buffer. After transfer to nylon membrane, the RNA was hybridized with a nick-translated cDNA probe [α-32P]dCTP, 3,000 Ci/mmol). Hybridization and washing of the blot were performed at high stringency. The membrane was hybridized first with the cDNA encoding the precursor DIM 2 peptide, then with a drosomycin cDNA probe (15), and finally with a ribosomal protein rp49 cDNA probe as control.
DISCUSSION
The data presented in this study demonstrate that MALDI-TOF MS is a powerful tool to analyze the consequences of an immune challenge in the hemolymph of a single fly. The way this technique was used here corresponds to a differential analysis, which allows the detection of inducible molecules without discriminating for a biological function, such as antimicrobial activity. In that respect, our approach is, conceptually at least, evocative of differential display PCR, a method that has yielded valuable results in the context of the immune response of Drosophila over the last 2 years (18). MALDI-TOF MS is not yet routinely applicable with high accuracy to molecules with masses above 11 kDa on samples of the complexity of hemolymph from a single fly, but we can anticipate that the present technical problems will be overcome in the foreseeable future.
The data obtained from hemolymph samples from control and immune-challenged wild-type or mutant flies call for the following comments.
Three inducible antimicrobial peptides could clearly be identified in the range of the masses that were analyzed in this study: drosomycin, metchnikowin, and drosocin.
The nondetection of cecropin A is related to problems inherent in the methodology, as explained in Results. The concentration of defensin in Drosophila is lower by one or two orders of magnitude than those of the other antimicrobial peptides, and was therefore not detected in the present study. Altogether, our results indicate that drosomycin, drosocin, and metchnikowin appear in the hemolymph 6 h after challenge, that their concentrations increase up to 24 h, and that they persist for unexpectedly long periods. Drosocin and metchnikowin remain detectable in the blood for 2 weeks and drosomycin remains for 3 weeks, whereas previous studies with Northern blots showed a decrease in transcriptional activities after 24–36 h (14, 15, 19). These relatively long periods of persistence of the antimicrobial peptides after their transcription has subsided indicates that they are particularly resistant to protease cleavage under in vivo conditions. This can be explained for drosomycin by its extremely compact three-dimensional structure (20). The changes in the relative ratios of the disaccharide and monosaccharide glycoforms of drosocin suggest that a circulating glycosidase first cleaves the distal sugar moiety before proteolytic cleavage.
The analysis of the induction of the identifiable antimicrobial peptides in various mutant backgrounds is in complete agreement with studies from this laboratory based on transcriptional profiles of the corresponding genes (2, 8, 21).
The new data confirm at the peptide level the paramount role of the Toll signaling pathway in the induction of the antifungal peptide drosomycin and that of the imd pathway for drosocin. They also confirm that metchnikowin can be induced both in Toll− and in imd mutants, in contrast to the other antimicrobial peptides whose induction is severely impaired in either of these mutants.
Our study revealed an unexpectedly large number of immune-induced peptides that have the same kinetics of appearance as the antimicrobial peptides.
Of particular interest is the observation that the induction of these molecules is dependent on either the Toll or the imd pathway, as is the case for the antimicrobial peptides. Significantly, 18 of these peptides are constitutively expressed in a Toll gain-of-function mutant background, as is the case for drosomycin. Such insects were not immune-challenged, which eliminates the possibility that any of the DIMs could be related to bacteria or to byproducts from injury. Only two DIMs are undetectable in immune-challenged imd mutants. The function of the unknown DIMs remains to be established. A requisite for this will be their full structural characterization, which can be achieved either by the methodology illustrated for DIMs 1, 2, and 4 in this study, or by tandem MS. As regards the latter strategy, either MALDI/postsource decay or nanoelectrospray MS/MS appear as possibilities. Finally, the molecular cloning experiments confirm that DIM 2 is synthesized by flies in response to a bacterial challenge, and they indicate that it is processed from a 46-amino acid precursor.
Acknowledgments
This study was supported by institutional grants from the Centre National de la Recherche Scientifique and the Université Louis Pasteur, of Strasbourg, France, and by grants from Human Frontiers in Science, Training and Mobility in Research, Association Française de Lutte contre la Mucoviscidose, and Rhône–Poulenc Agro. The financial support of BioAvenir (Rhône–Poulenc Santé) is gratefully acknowledged by M.M. and A.V.D.
ABBREVIATIONS
- MALDI
matrix-assisted laser desorption/ionization
- TOF
time-of-flight
- DIM
Drosophila immune-induced molecule
- TFA
trifluoroacetic acid
- 4HCCA
α-cyano-4-hydroxycinnamic acid
- NC
nitrocellulose
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF074003).
References
- 1.Hoffmann J A, Reichhart J-M. Trends Cell Biol. 1997;7:309–316. doi: 10.1016/S0962-8924(97)01087-8. [DOI] [PubMed] [Google Scholar]
- 2.Lemaitre B, Nicolas E, Michaut L, Reichhart J-M, Hoffmann J A. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 3.Siuzdak G. Proc Natl Acad Sci USA. 1994;91:11290–11297. doi: 10.1073/pnas.91.24.11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roepstorff P. Curr Opin Biotechnol. 1997;8:6–13. doi: 10.1016/s0958-1669(97)80151-6. [DOI] [PubMed] [Google Scholar]
- 5.Vorm O, Chait B T, Roepstorff P. Proceedings of the 41st ASMS Conference on Mass Spectrometry and Allied Topics. San Francisco, CA: American Society for Mass Spectrometry; 1993. p. 621. [Google Scholar]
- 6.Uttenweiler-Joseph S, Moniatte M, Lambert J, Van Dorsselaer A, Bulet P. Anal Biochem. 1997;247:366–375. doi: 10.1006/abio.1997.2083. [DOI] [PubMed] [Google Scholar]
- 7.Jimenez C R, Li K W, Dreisewerd K, Mansvelder H D, Brussaard A B, Reinhold B B, Van der Schors R C, Karas M, Hillenkamp F, Burbach J P H, Costello C E, et al. Proc Natl Acad Sci USA. 1997;94:9481–9486. doi: 10.1073/pnas.94.17.9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemaitre B, Kromer-Metzger E, Michaut L, Nicolas E, Meister M, Georgel P, Reichhart J-M, Hoffmann J A. Proc Natl Acad Sci USA. 1995;92:9465–9469. doi: 10.1073/pnas.92.21.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vorm O, Roepstorff P, Mann M. Anal Chem. 1994;66:3281–3287. [Google Scholar]
- 10.Kussmann M, Nordhoff E, Rahbek-Nielsen H, Haebel S, Rossel-Larsen M, Jakobsen L, Gobom J, Mirgorodskaya E, Kroll-Kristensen A, Palm L, et al. J Mass Spectrom. 1997;32:593–601. [Google Scholar]
- 11.Jensen O N, Mortensen P, Vorm O, Mann M. Anal Chem. 1997;69:1706–1714. doi: 10.1021/ac961189t. [DOI] [PubMed] [Google Scholar]
- 12.Bulet P, Dimarcq J-L, Hetru C, Lagueux M, Charlet M, Hegy G, Van Dorsselaer A, Hoffmann J A. J Biol Chem. 1993;20:14893–14897. [PubMed] [Google Scholar]
- 13.Bulet P, Urge L, Ohresser S, Hetru C, Otvos L., Jr Eur J Biochem. 1996;238:64–69. doi: 10.1111/j.1432-1033.1996.0064q.x. [DOI] [PubMed] [Google Scholar]
- 14.Levashina E A, Ohresser S, Bulet P, Reichhart J-M, Hetru C, Hoffmann J A. Eur J Biochem. 1995;233:694–700. doi: 10.1111/j.1432-1033.1995.694_2.x. [DOI] [PubMed] [Google Scholar]
- 15.Fehlbaum P, Bulet P, Michaut L, Lagueux M, Broekaert W F, Hetru C, Hoffmann J A. J Biol Chem. 1994;52:33159–33163. [PubMed] [Google Scholar]
- 16.Dimarcq J-L, Hoffmann D, Meister M, Bulet P, Lanot R, Reichhart J-M, Hoffmann J A. Eur J Biochem. 1994;221:201–209. doi: 10.1111/j.1432-1033.1994.tb18730.x. [DOI] [PubMed] [Google Scholar]
- 17.Boman H G. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 18.Dushay M S, Asling B, Hultmark D. Proc Natl Acad Sci USA. 1996;93:10343–10347. doi: 10.1073/pnas.93.19.10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlet M, Lagueux M, Reichhart J-M, Hoffmann D, Braun A, Meister M. Eur J Biochem. 1996;241:699–706. doi: 10.1111/j.1432-1033.1996.00699.x. [DOI] [PubMed] [Google Scholar]
- 20.Landon C, Sodano P, Hetru C, Hoffmann J A, Ptak M. Protein Sci. 1997;6:1878–1884. doi: 10.1002/pro.5560060908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levashina E A, Ohresser S, Lemaitre B, Imler J-L. J Mol Biol. 1998;278:515–527. doi: 10.1006/jmbi.1998.1705. [DOI] [PubMed] [Google Scholar]