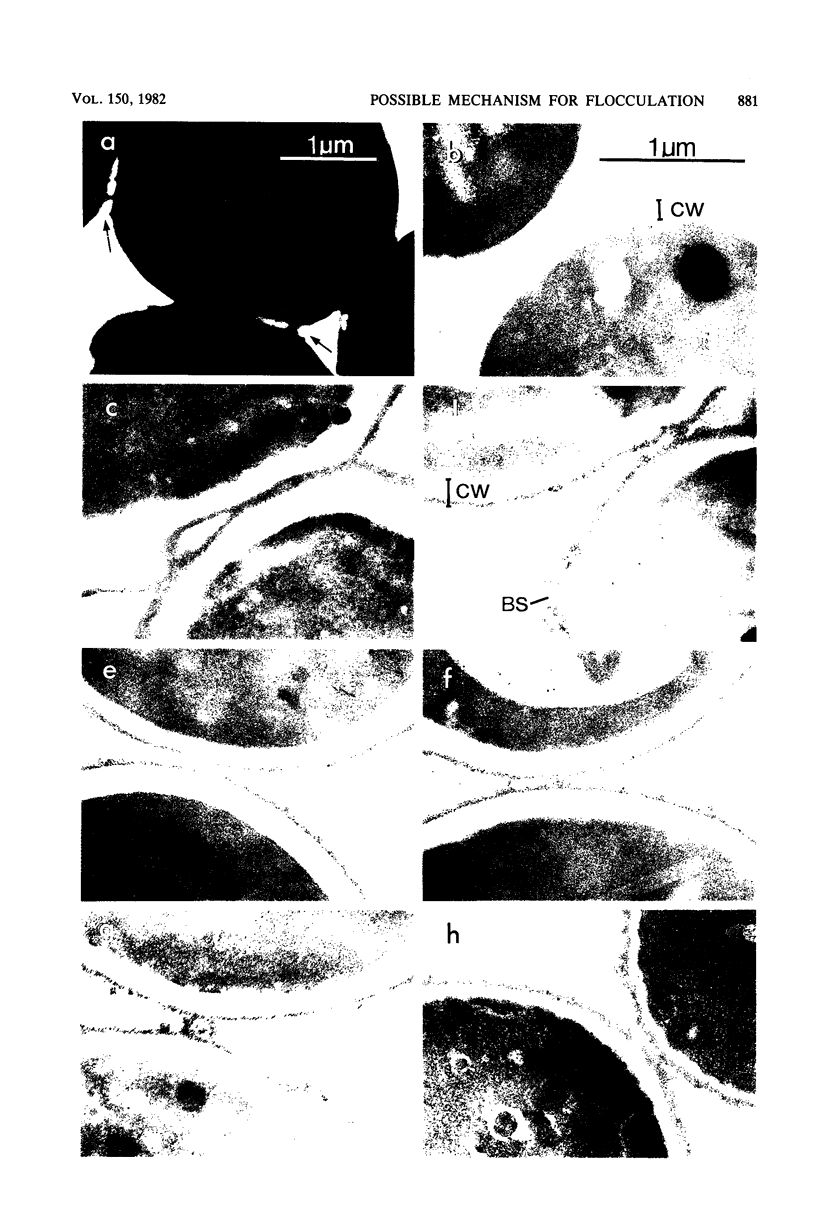
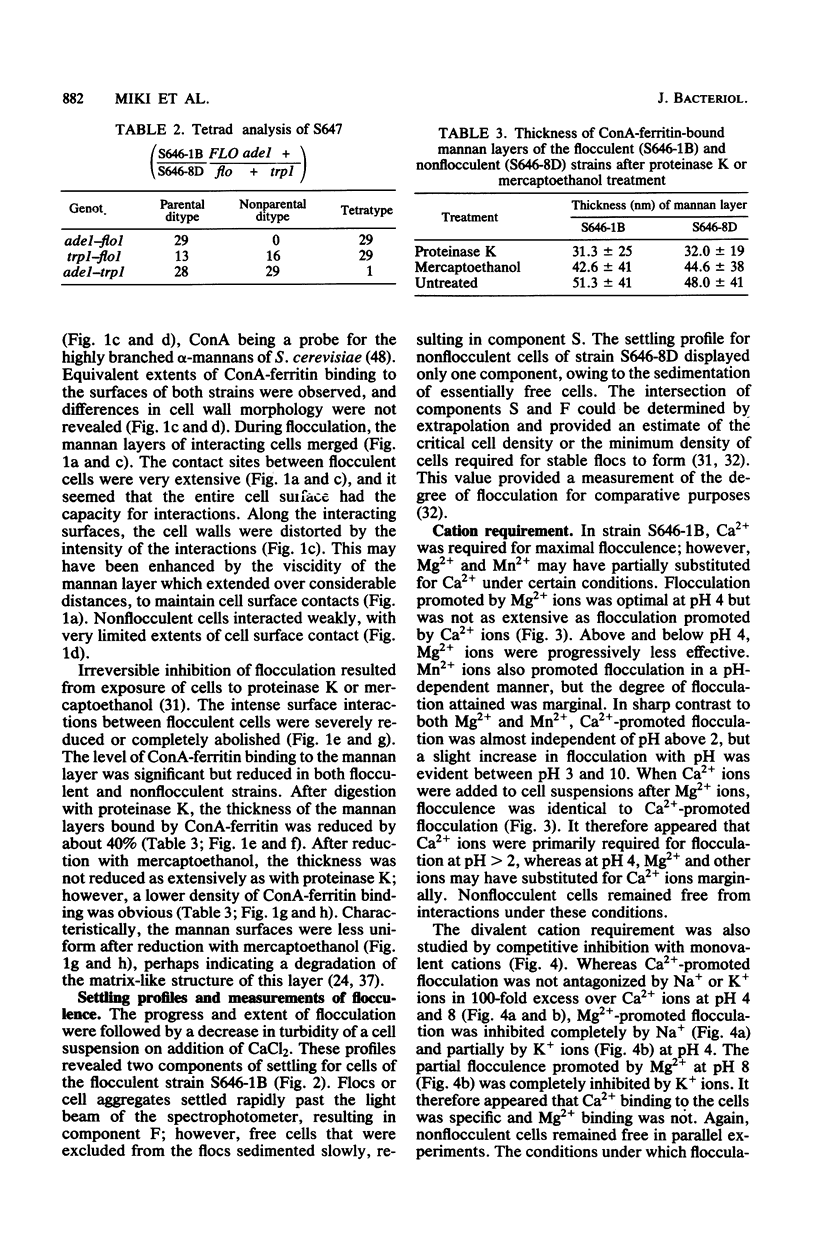
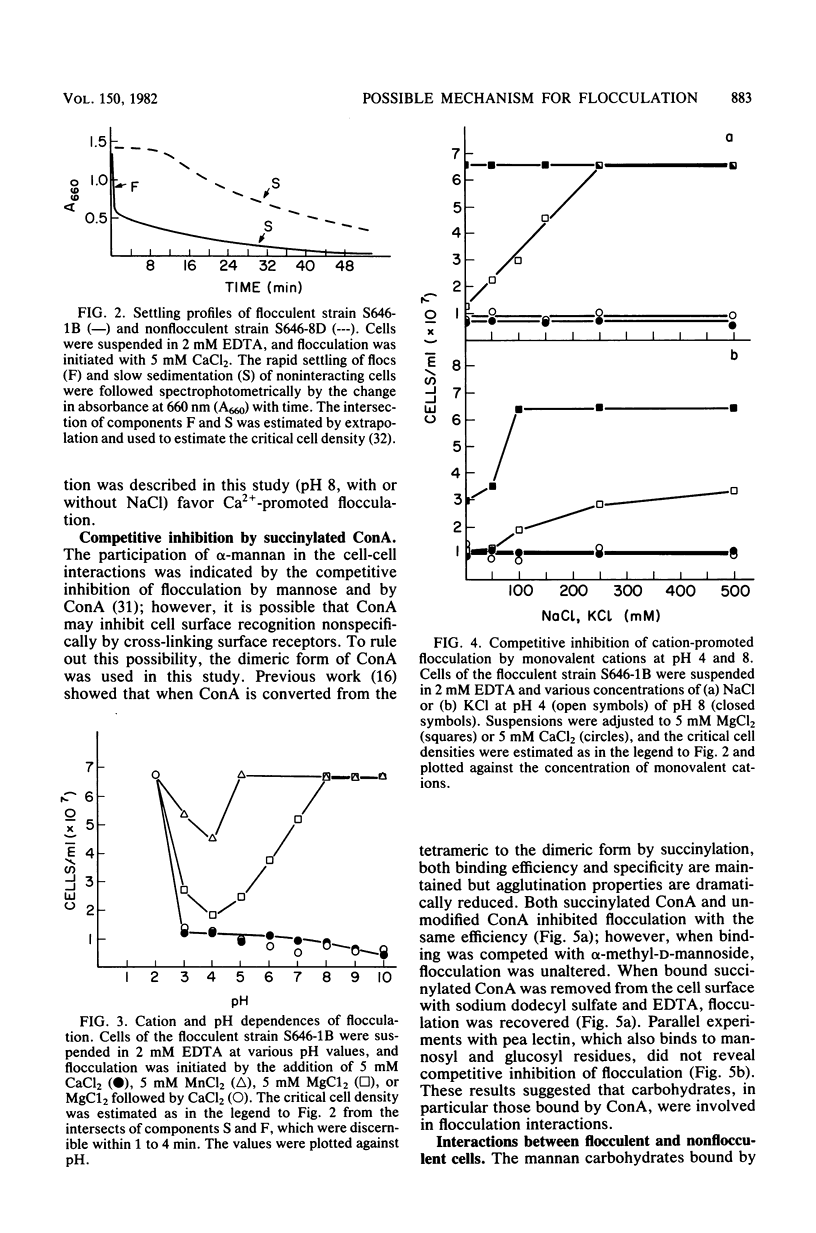
Abstract
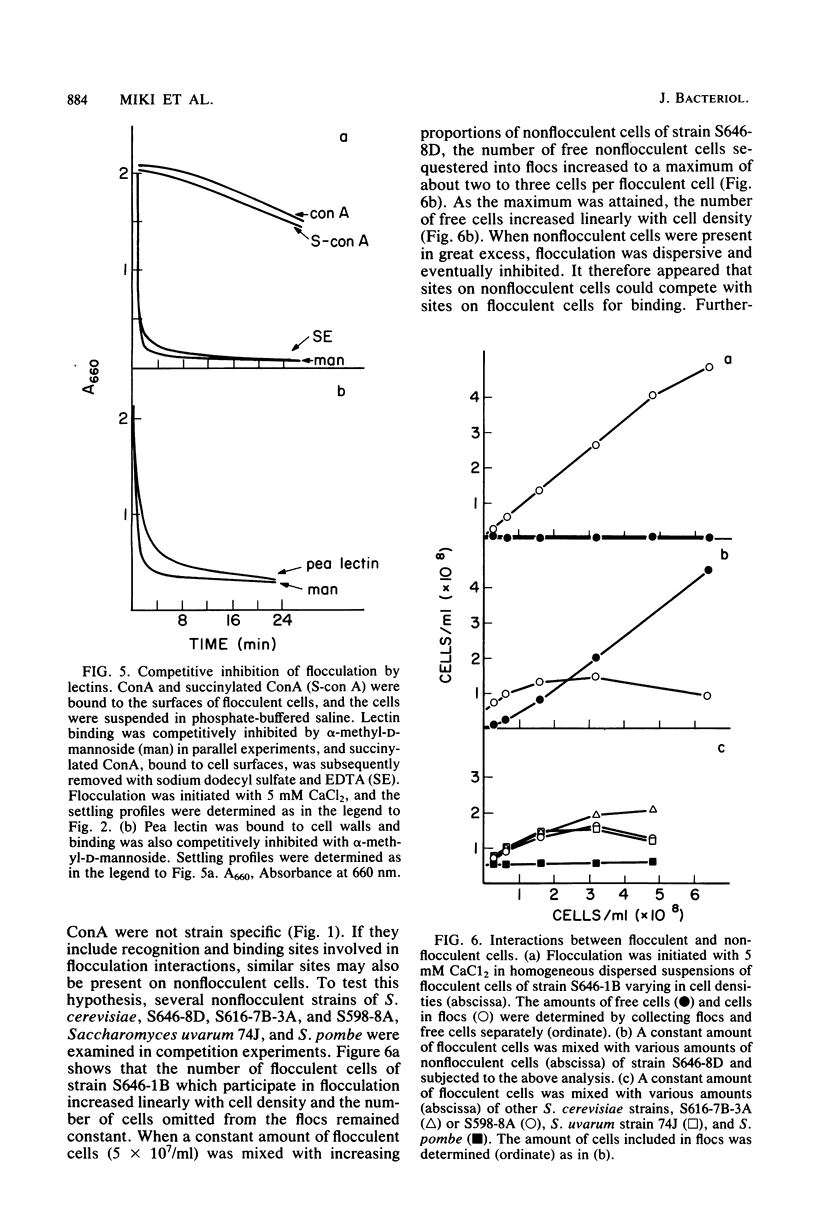
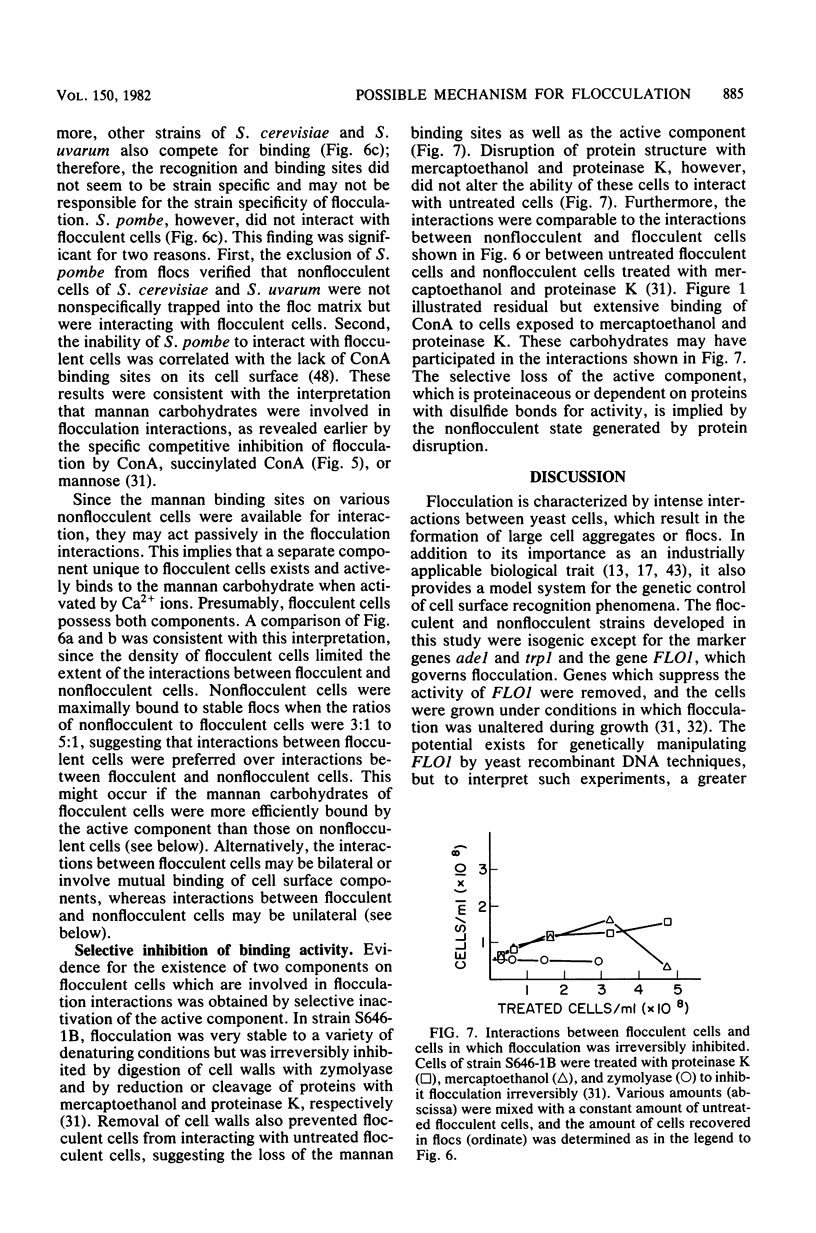
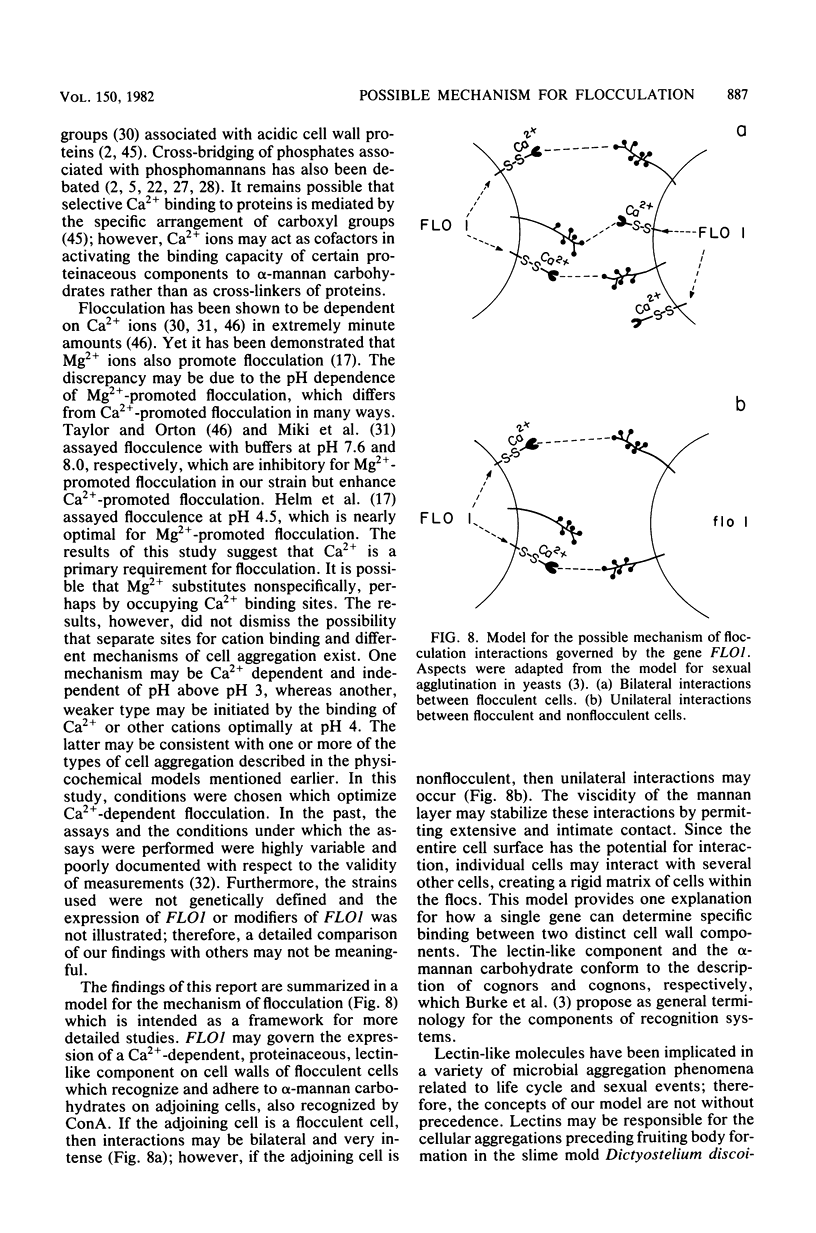
A model is proposed for the mechanism of flocculation interactions in yeasts in which flocculent cells have a recognition factor which attaches to alpha-mannan sites on other cells. This factor may be governed by the expression of the single, dominant gene FLO1. Isogenic strains of Saccharomyces cerevisiae, differing only at FLO1 and the marker genes ade1 and trp1, were developed to examine the components involved in flocculene. Electron microscopy and concanavalin Aferritin labeling of aggregated cells showed that extensive and intense interactions between cell wall mannan layers mediated cell aggregation. The components of the mannan layer essential for flocculence were Ca2+ ions, alpha-mannan carbohydrates, and proteins. By studying the divalent cation dependence at various pH values and in the presence of competing monovalent cations, flocculation was found to be Ca2+ dependent; however, Mg2+ and Mn2+ ions substituted for Ca2+ under certain conditions. Reversible inhibition of flocculation by concanavalin A and succinylated concanavalin A implicated alpha-branched mannan carbohydrates as one essential component which alone did not determine the strain specificity of flocculence, since nonflocculent strains interacted with and competed for binding sites on flocculent cells. FLO1 may govern the expression of a proteinaceous, lectin-like activity, firmly associated with the cell walls of flocculent cells, which bind to the alpha-mannan carbohydrates of adjoining cells. It was selectively and irreversibly inhibited by proteolysis and reduction of disulfide bonds. The potential of this system as a model for the genetic and biochemical control of cell-cell interactions is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou C. E., Raschke W. C. Polymorphism of the somatic antigen of yeast. Science. 1974 Apr 12;184(4133):127–134. doi: 10.1126/science.184.4133.127. [DOI] [PubMed] [Google Scholar]
- Beavan M. J., Belk D. M., Stewart G. G., Rose A. H. Changes in electrophoretic mobility and lytic enzyme activity associated with development of flocculating ability in Saccharomyces cerevisiae. Can J Microbiol. 1979 Aug;25(8):888–895. doi: 10.1139/m79-132. [DOI] [PubMed] [Google Scholar]
- Burke D., Mendonça-Previato L., Ballou C. E. Cell-cell recognition in yeast: purification of Hansenula wingei 21-cell sexual agglutination factor and comparison of the factors from three genera. Proc Natl Acad Sci U S A. 1980 Jan;77(1):318–322. doi: 10.1073/pnas.77.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley T. N., Ballou C. E. Identification of two Saccharomyces cerevisiae cell wall mannan chemotypes. J Bacteriol. 1972 Sep;111(3):690–695. doi: 10.1128/jb.111.3.690-695.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall M. Mating-type interactions in yeasts. Symp Soc Exp Biol. 1978;32:105–120. [PubMed] [Google Scholar]
- Cumsky M., Zusman D. R. Myxobacterial hemagglutinin: a development-specific lectin of Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5505–5509. doi: 10.1073/pnas.76.11.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day A. W., Poon N. H. Fungal fimbriae. II. Their role in conjugation in Ustilago violacea. Can J Microbiol. 1975 Apr;21(4):547–557. doi: 10.1139/m75-077. [DOI] [PubMed] [Google Scholar]
- Day A. W., Poon N. H., Stewart G. G. Fungal fimbriae. III. The effect on flocculation in Saccharomyces. Can J Microbiol. 1975 Apr;21(4):558–564. doi: 10.1139/m75-078. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Yanke W. E., Brill W. J. Trifolin: a Rhizobium recognition protein from white clover. Biochim Biophys Acta. 1978 Mar 20;539(3):276–286. doi: 10.1016/0304-4165(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Geltosky J. E., Weseman J., Bakke A., Lerner R. A. Identification of a cell surface glycoprotein involved in cell aggregation in D. discoideum. Cell. 1979 Oct;18(2):391–398. doi: 10.1016/0092-8674(79)90058-8. [DOI] [PubMed] [Google Scholar]
- Grabel L. B., Rosen S. D., Martin G. R. Teratocarcinoma stem cells have a cell surface carbohydrate-binding component implicated in cell-cell adhesion. Cell. 1979 Jul;17(3):477–484. doi: 10.1016/0092-8674(79)90255-1. [DOI] [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Yahara I., Cunningham B. A., Edelman G. M. Concanavalin A derivatives with altered biological activities. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1012–1016. doi: 10.1073/pnas.70.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J., Strathern J. N., Klar A. J. Transposable mating type genes in Saccharomyces cerevisiae. Nature. 1979 Nov 29;282(5738):478–473. doi: 10.1038/282478a0. [DOI] [PubMed] [Google Scholar]
- Horisberger M., Vonlanthen M. Location of mannan and chitin on thin sections of budding yeasts with gold markers. Arch Microbiol. 1977 Oct 24;115(1):1–7. doi: 10.1007/BF00427837. [DOI] [PubMed] [Google Scholar]
- Jayatissa P. M., Rose A. H. Role of wall phosphomannan in flocculation of Saccharomyces cerevisiae. J Gen Microbiol. 1976 Sep;96(1):165–174. doi: 10.1099/00221287-96-1-165. [DOI] [PubMed] [Google Scholar]
- Kubota T., Tokoroyama T., Tsukuda Y., Koyama H., Miyake A. Isolation and structure determination of blepharismin, a conjugation initiating gamone in the ciliate blepharisma. Science. 1973 Jan 26;179(4071):400–402. doi: 10.1126/science.179.4071.400. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampen J. O. External enzymes of yeast: their nature and formation. Antonie Van Leeuwenhoek. 1968;34(1):1–18. doi: 10.1007/BF02046409. [DOI] [PubMed] [Google Scholar]
- Lipke P. N., Taylor A., Ballou C. E. Morphogenic effects of alpha-factor on Saccharomyces cerevisiae a cells. J Bacteriol. 1976 Jul;127(1):610–618. doi: 10.1128/jb.127.1.610-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILL P. J. THE NATURE OF THE INTERACTIONS BETWEEN FLOCCULENT CELLS IN THE FLOCCULATION OF SACCHAROMYCES CEREVISIAE. J Gen Microbiol. 1964 Apr;35:61–68. doi: 10.1099/00221287-35-1-61. [DOI] [PubMed] [Google Scholar]
- Miki B. L., Poon N. H., Seligy V. L. Repression and induction of flocculation interactions in Saccharomyces cerevisiae. J Bacteriol. 1982 May;150(2):890–899. doi: 10.1128/jb.150.2.890-899.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara H., Toraya T., Fukui S. Effect of chemical modification of cell surface components of a brewer's yeast on the floc-forming ability. Arch Microbiol. 1977 Oct 24;115(1):19–23. doi: 10.1007/BF00427840. [DOI] [PubMed] [Google Scholar]
- Nowak T. P., Haywood P. L., Barondes S. H. Developmentally regulated lectin in embryonic chick muscle and a myogenic cell line. Biochem Biophys Res Commun. 1976 Feb 9;68(3):650–657. doi: 10.1016/0006-291x(76)91195-5. [DOI] [PubMed] [Google Scholar]
- Osumi M., Shimoda C., Yanagishima N. Mating reaction in Saccharomyces cerevisiae. V. Changes in the fine structure during the mating reaction. Arch Mikrobiol. 1974 Apr 10;97(1):27–38. [PubMed] [Google Scholar]
- Rosen S. D., Kafka J. A., Simpson D. L., Barondes S. H. Developmentally regulated, carbohydrate-binding protein in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2554–2557. doi: 10.1073/pnas.70.9.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowekamp W., Poole S., Firtel R. A. Analysis of the multigene family coding the developmentally regulated carbohydrate-binding protein discoidin-I in D. discoideum. Cell. 1980 Jun;20(2):495–505. doi: 10.1016/0092-8674(80)90636-4. [DOI] [PubMed] [Google Scholar]
- Simpson D. L., Thorne D. R., Loh H. H. Lectins: endogenous carbohydrate-binding proteins from vertebrate tissues: functional role in recognition processes? Life Sci. 1978 Mar;22(9):727–748. doi: 10.1016/0024-3205(78)90242-4. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stewart G. G., Russell I. The identification, characterization, and mapping of a gene for flocculation in Saccharomyces sp. Can J Microbiol. 1977 Apr;23(4):441–447. doi: 10.1139/m77-065. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Cybulska E. B., Lampen J. O. Specific staining of wall mannan in yeast cells with fluorescein-conjugated concanavalin A. J Bacteriol. 1971 Jan;105(1):1–5. doi: 10.1128/jb.105.1.1-5.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacz J. S., MacKay V. L. Sexual conjugation in yeast. Cell surface changes in response to the action of mating hormones. J Cell Biol. 1979 Feb;80(2):326–333. doi: 10.1083/jcb.80.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]