Abstract
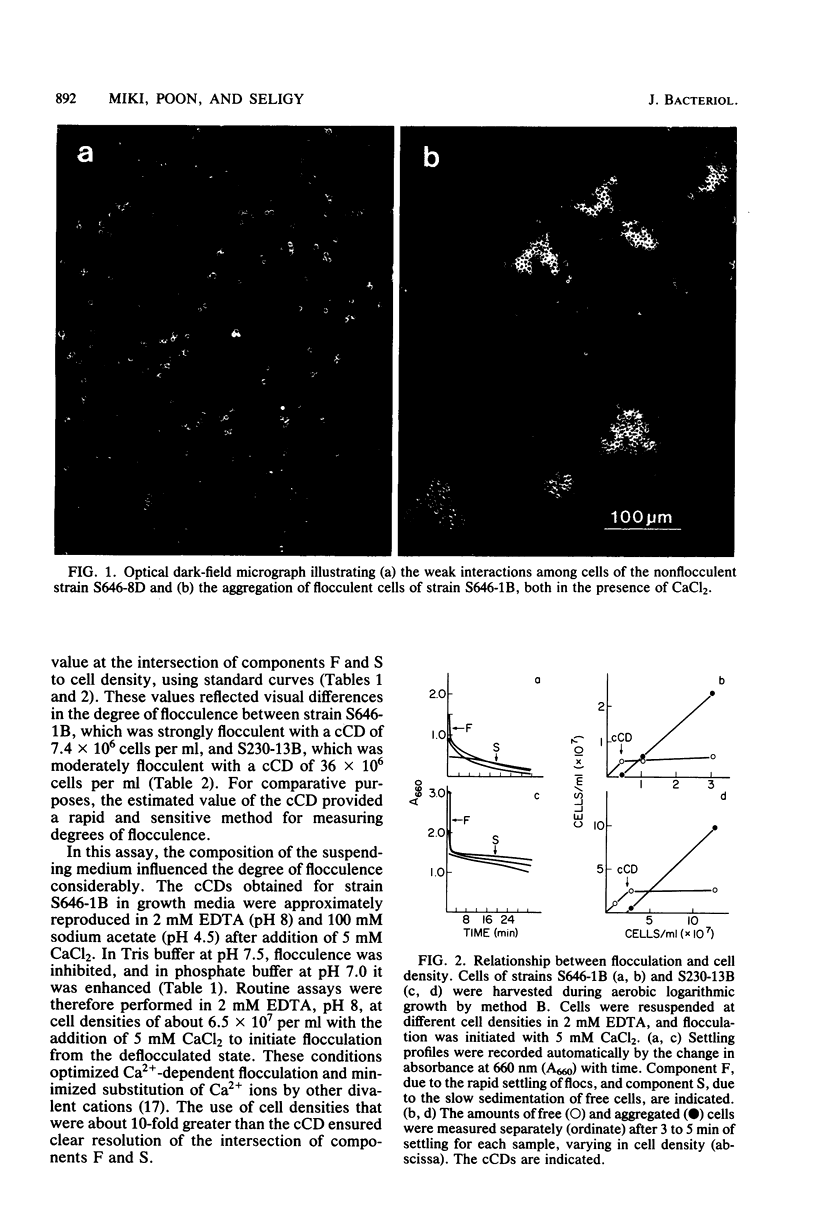
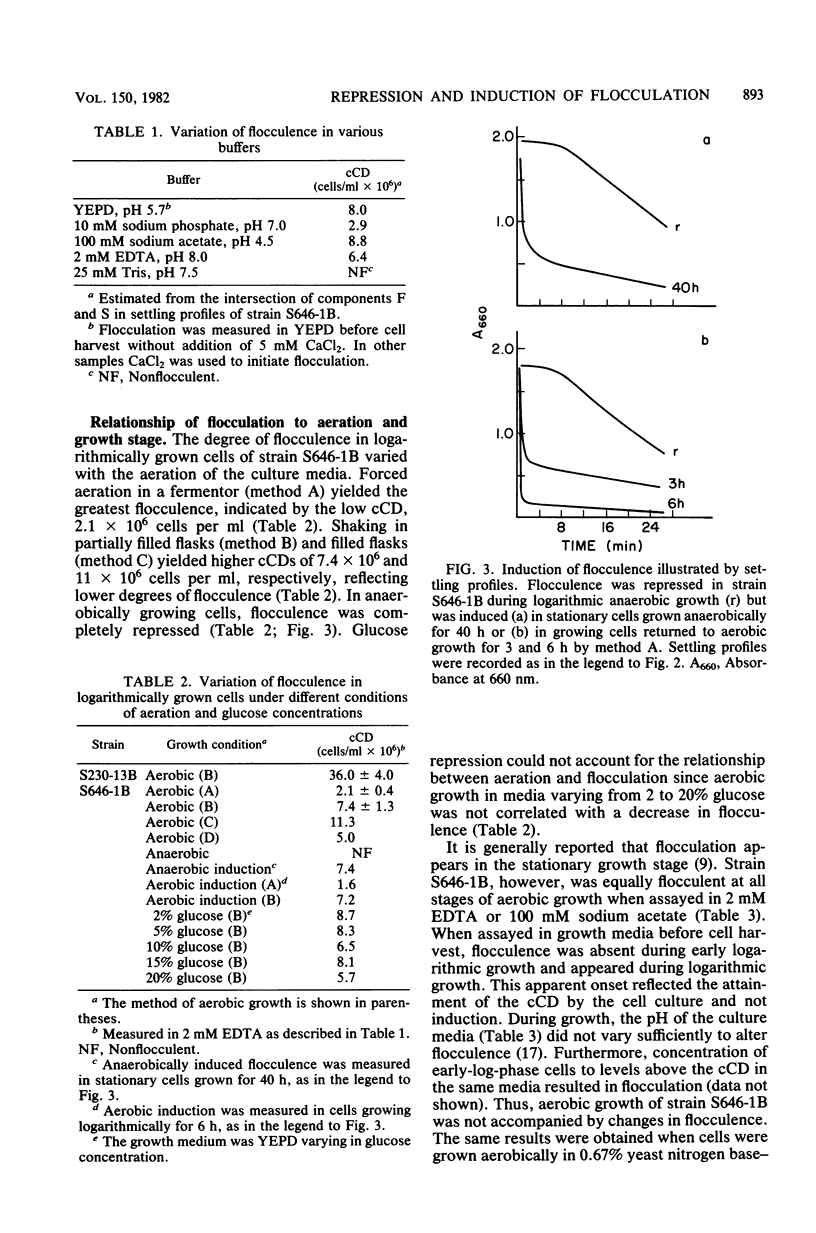
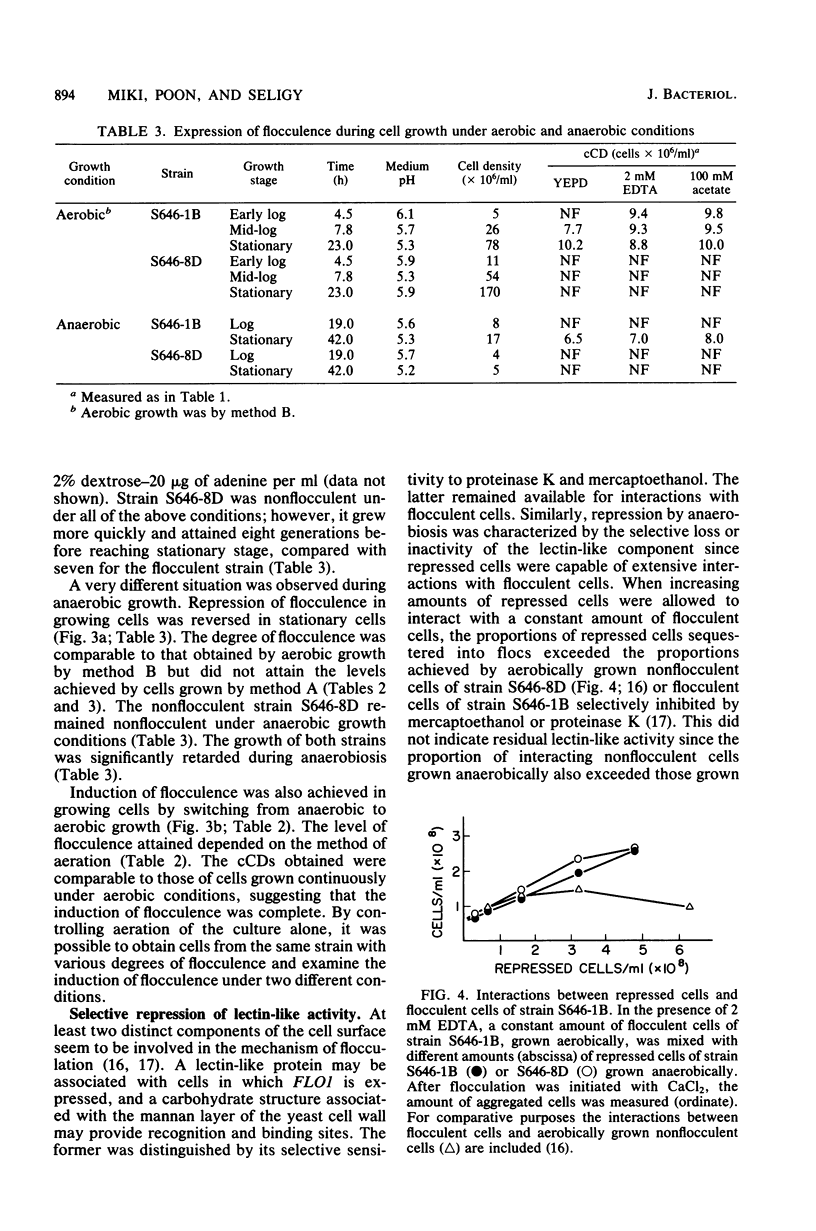
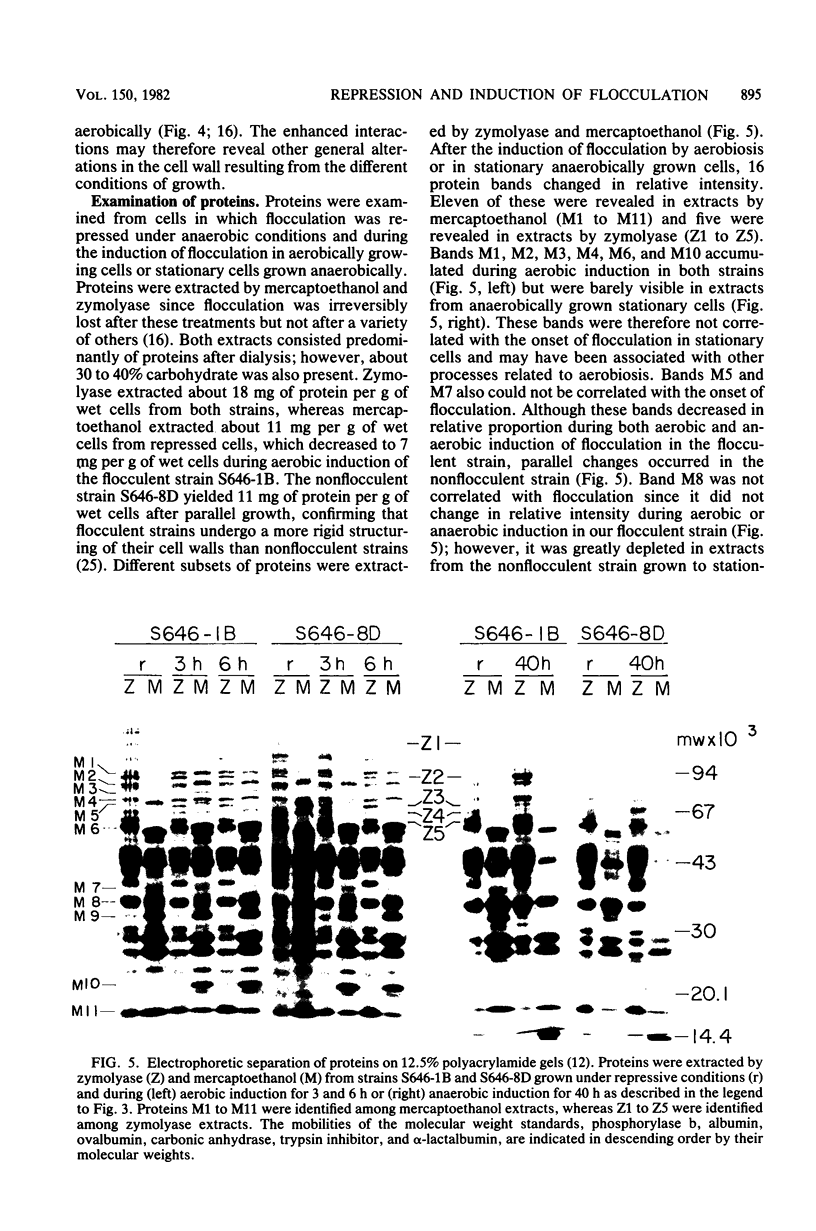
The biological control of flocculation interactions by factors related to growth under different conditions of aeration was documented with a new assay for flocculence. The degree of flocculence expressed in a genetically defined Saccharomyces cerevisiae strain (FLO1/FLO1 ade1/ade1) remained constant during aerobic growth but varied with aeration. Flocculence was repressed in anaerobically growing cells but was induced in stationary cells or cells returned to aerobic growth. Repression was correlated with the selective inactivation of cell surface lectin-like components. The changes in flocculence were accompanied by changes in 16 extractable proteins separated by electrophoresis; however, a clear correlation between specific protein bands and flocculence could not be established. The study clearly demonstrated that the phenotypic expression of FLO1 could be reproducibly manipulated for experimental purposes by aeration alone.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavan M. J., Belk D. M., Stewart G. G., Rose A. H. Changes in electrophoretic mobility and lytic enzyme activity associated with development of flocculating ability in Saccharomyces cerevisiae. Can J Microbiol. 1979 Aug;25(8):888–895. doi: 10.1139/m79-132. [DOI] [PubMed] [Google Scholar]
- Calleja G. B., Johnson B. F. Temperature sensitivity of flocculation induction, conjugation and sporulation in fission yeast. Antonie Van Leeuwenhoek. 1979;45(3):391–400. doi: 10.1007/BF00443278. [DOI] [PubMed] [Google Scholar]
- Calleja G. B. On the nature of the forces involved in the sex-directed flocculation of a fission yeast. Can J Microbiol. 1974 Jun;20(6):797–803. doi: 10.1139/m74-123. [DOI] [PubMed] [Google Scholar]
- Calleja G. B. Role of mitochondria in the sex-directed flocculation of a fission yeast. Arch Biochem Biophys. 1973 Jan;154(1):382–386. doi: 10.1016/0003-9861(73)90070-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MILL P. J. THE EFFECT OF NITROGENOUS SUBSTANCES ON THE TIME OF FLOCCULATION OF SACCHAROMYCES CEREVISIAE. J Gen Microbiol. 1964 Apr;35:53–60. doi: 10.1099/00221287-35-1-53. [DOI] [PubMed] [Google Scholar]
- Nishihara H., Toraya T., Fukui S. Effect of chemical modification of cell surface components of a brewer's yeast on the floc-forming ability. Arch Microbiol. 1977 Oct 24;115(1):19–23. doi: 10.1007/BF00427840. [DOI] [PubMed] [Google Scholar]
- Stewart G. G., Russell I. The identification, characterization, and mapping of a gene for flocculation in Saccharomyces sp. Can J Microbiol. 1977 Apr;23(4):441–447. doi: 10.1139/m77-065. [DOI] [PubMed] [Google Scholar]




