Abstract
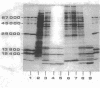
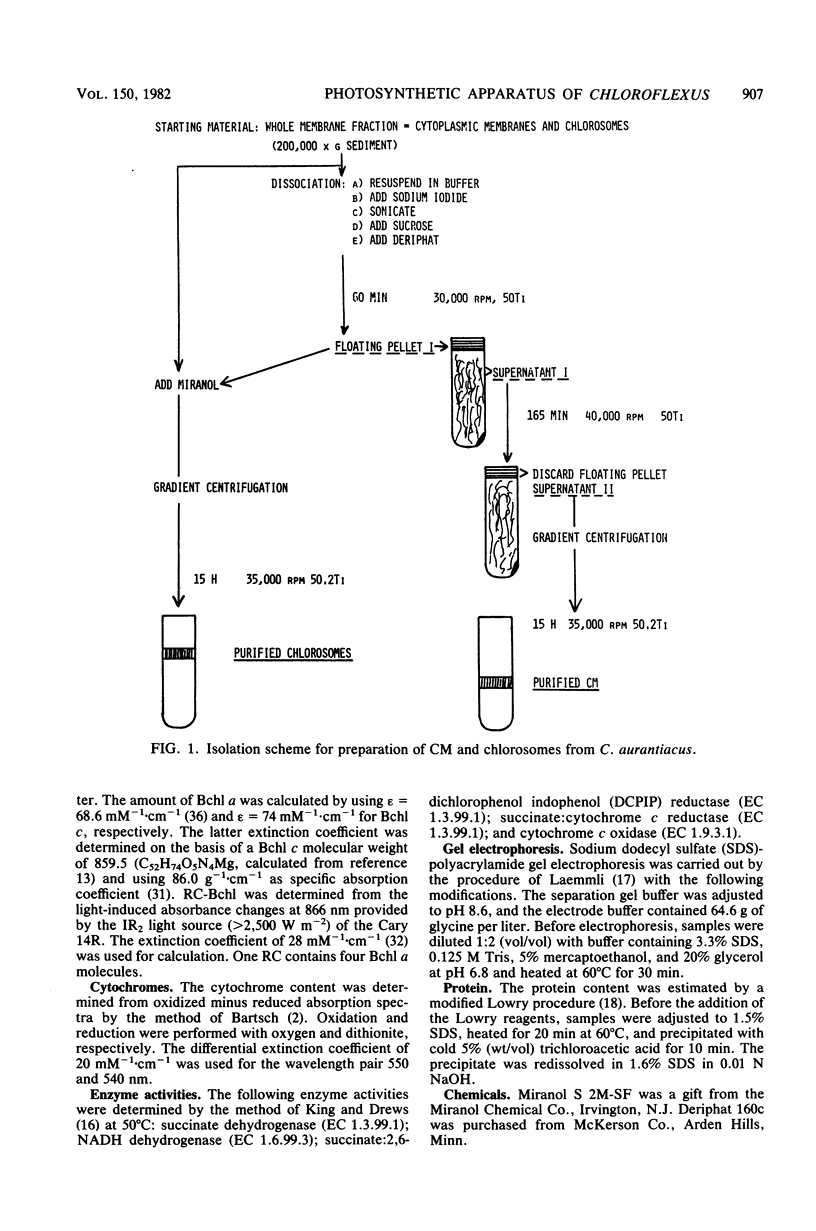
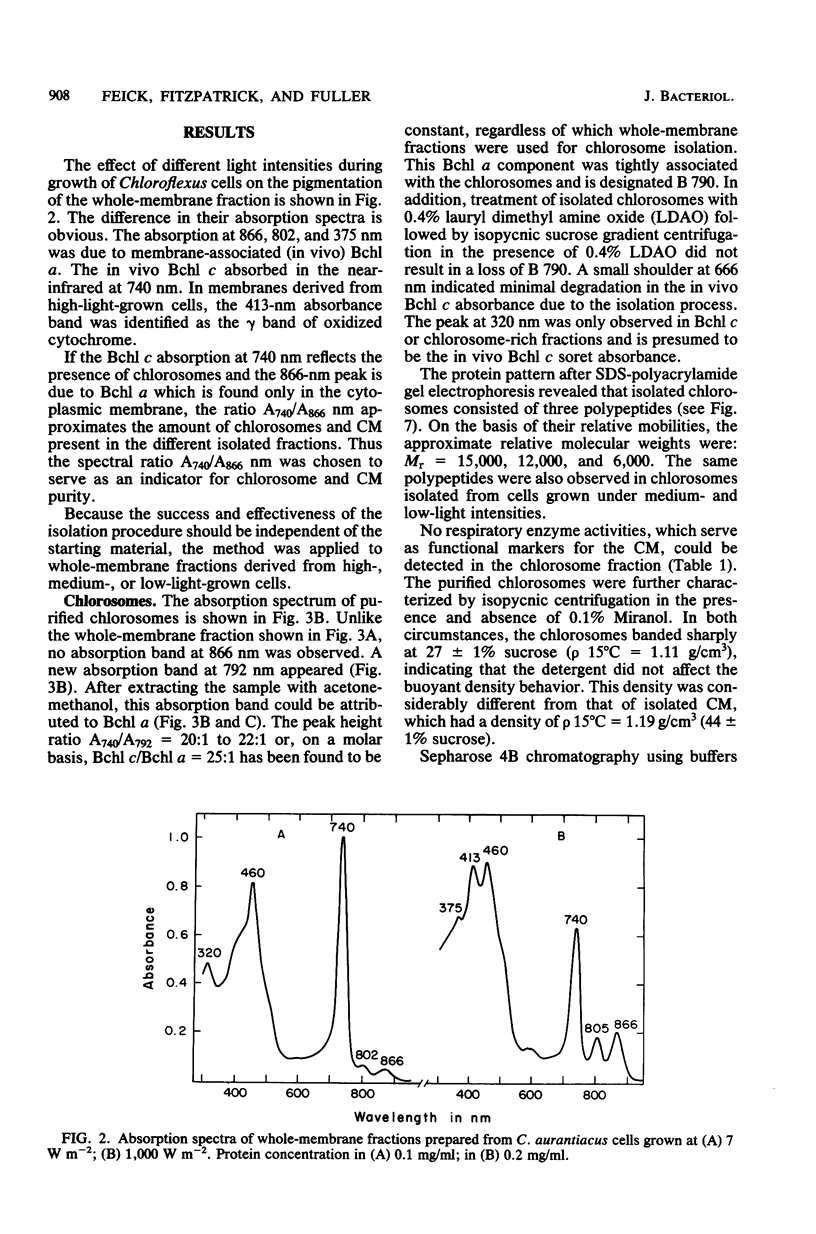
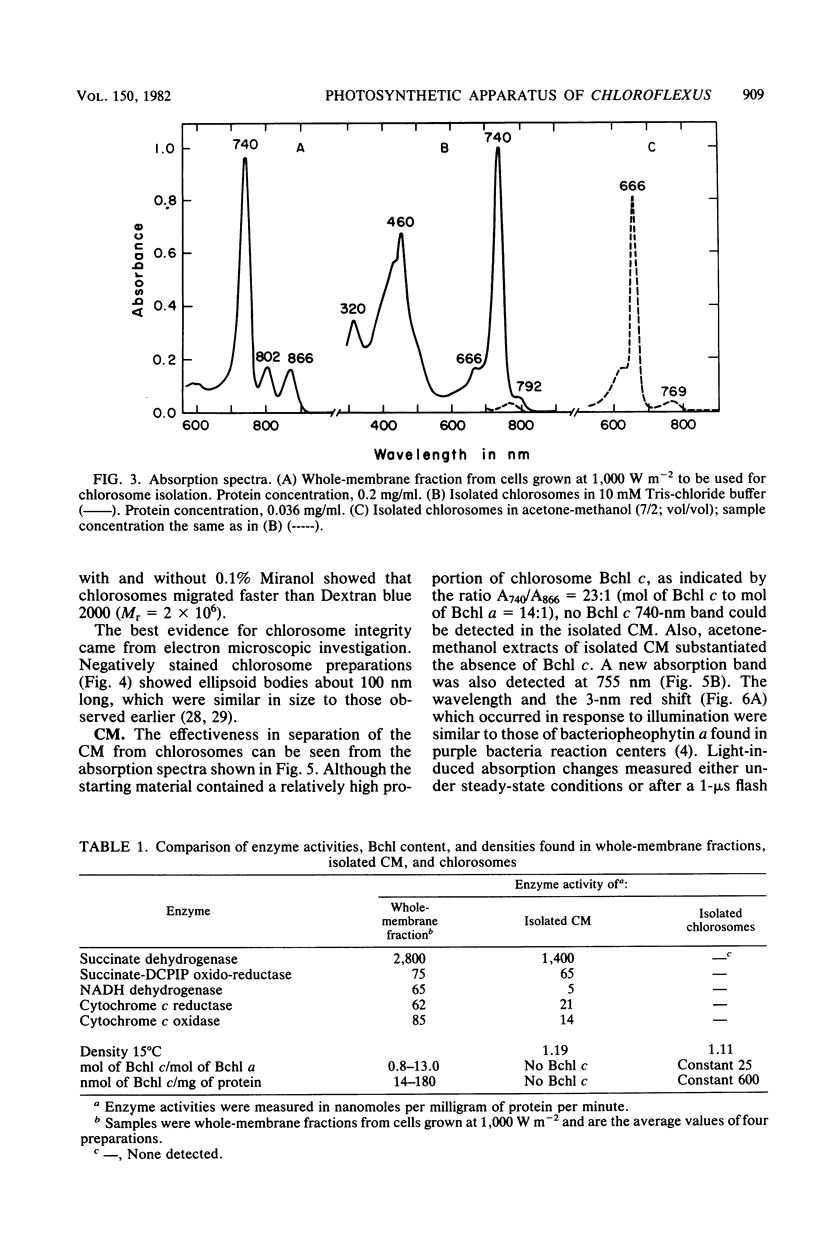
A method was developed which allows the isolation and purification of cytoplasmic membranes and chlorosomes from cells of Chloroflexus aurantiacus grown under different light conditions. The dipolar ionic detergent Deriphat (0.08%) and a sodium iodide gradient centrifugation were used in isolating cytoplasmic membranes. Chlorosomes were prepared with 0.16% of the dipolar ionic detergent Miranol and purified by a sucrose gradient centrifugation. Cytoplasmic membrane fractions prepared from either high- (3,000 W m-2), medium-(200 W m-2) or low- (7 W m-2) light-grown cells had near infrared absorption bands at 866, 808, and 755 nm in a constant characteristic absorbance ratio of 6:3.8:1. In all cytoplasmic membrane preparations, the amount of bacteriochlorophyll a (Bchl a) per cytochrome, the amount of Bchl a per reaction center, and reaction center per milligram of cytoplasmic membrane protein was found to be constant. No Bchl c was present. Five respiratory enzyme activities have been measured in the cytoplasmic membrane fraction. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of denatured cytoplasmic membrane showed many bands, but a major polypeptide with an apparent molecular weight of 8,000. In contrast, sodium dodecyl sulfate-polyacrylamide gel electrophoresis of purified chlorosomes did not contain the 8,000-molecular-weight band but revealed only three distinct protein bands with molecular weights of 15,000, 12,000, and 6,000. Isolated chlorosomes contained Bchl c and a small, yet constant, amount of Bchl a (absorbing at 790 nm) in a molar ratio of 25:1. The data indicated that the components of the photosynthetic apparatus in the cytoplasmic membrane of Chloroflexus aurantiacus remained constant and only the amount of antenna Bchl c varied with light conditions.
Full text
PDF

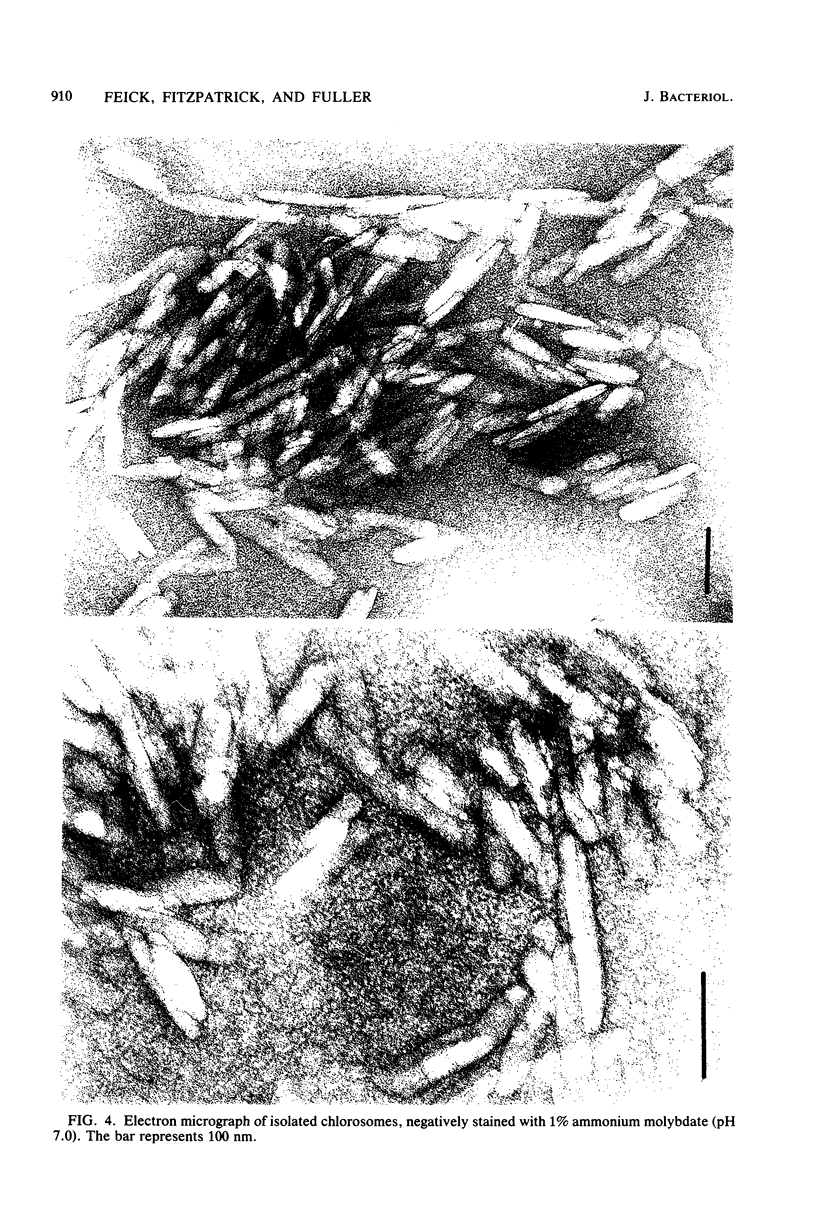
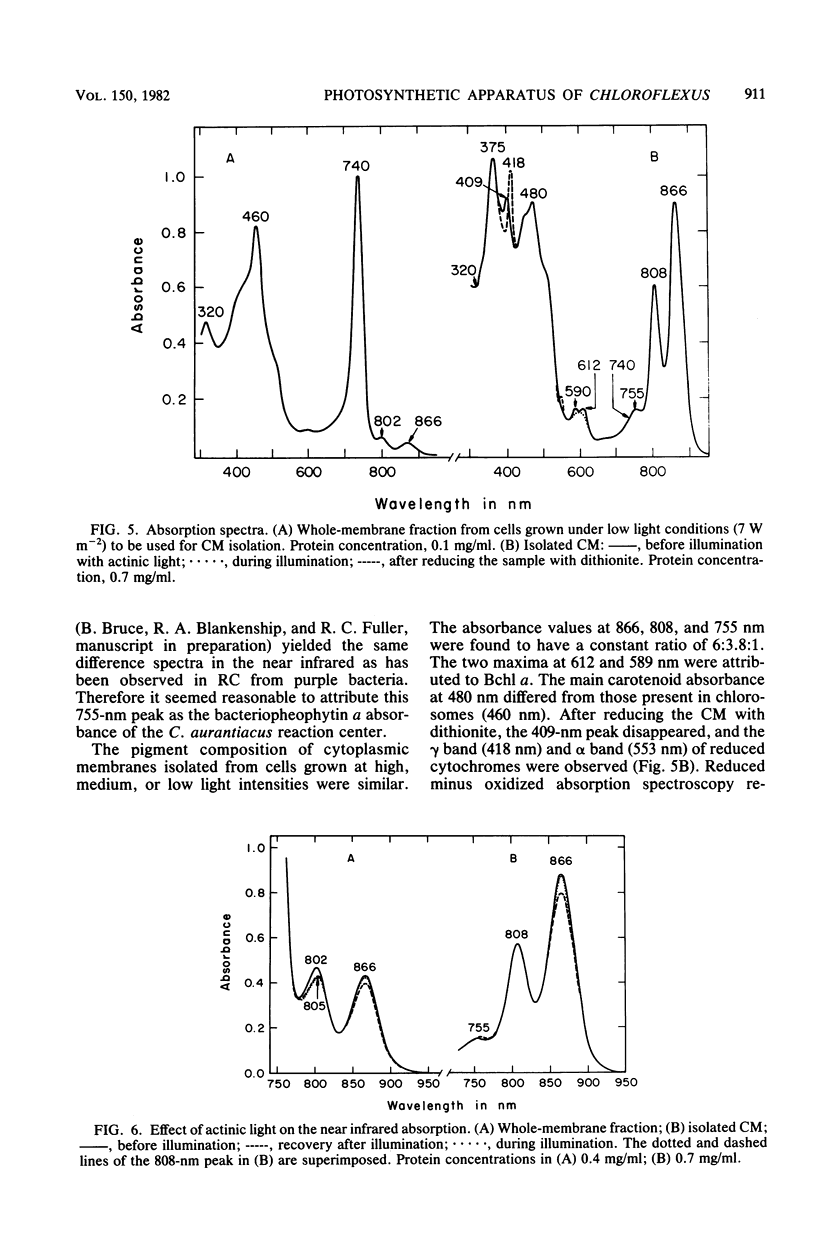
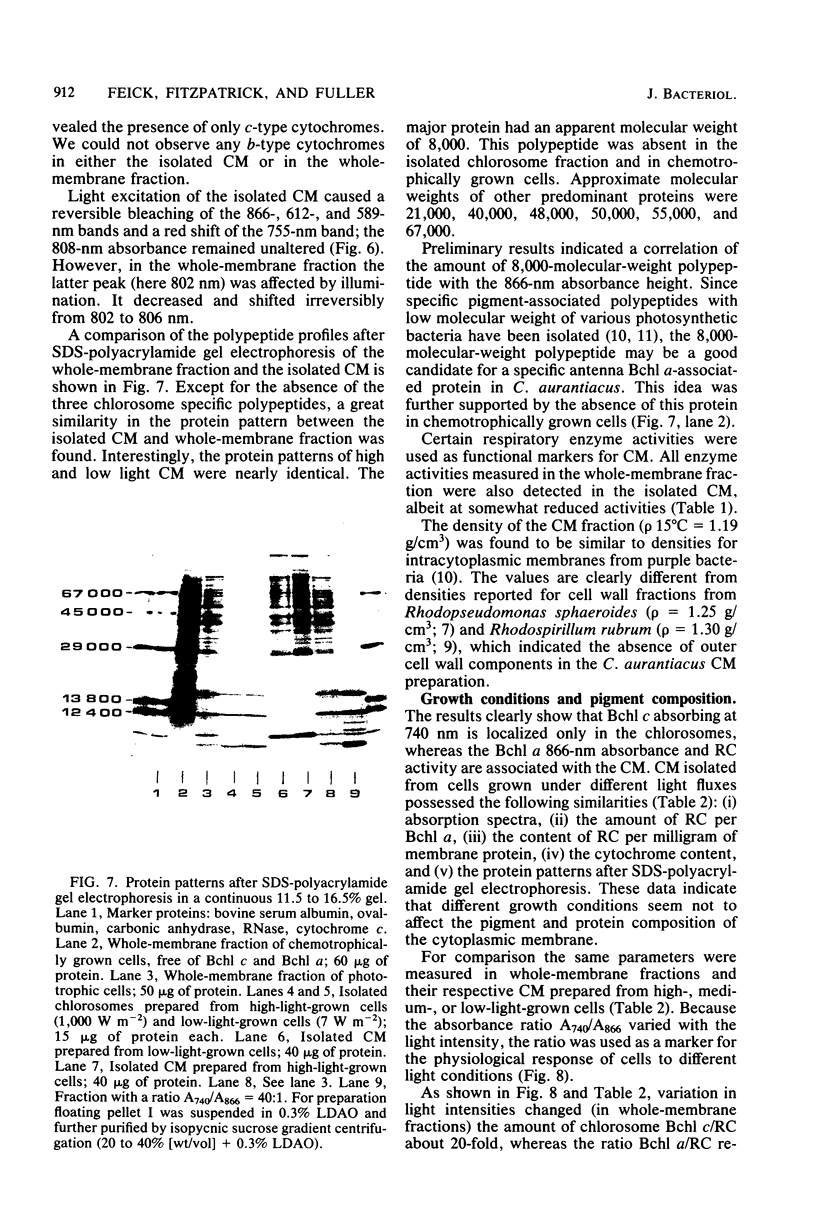
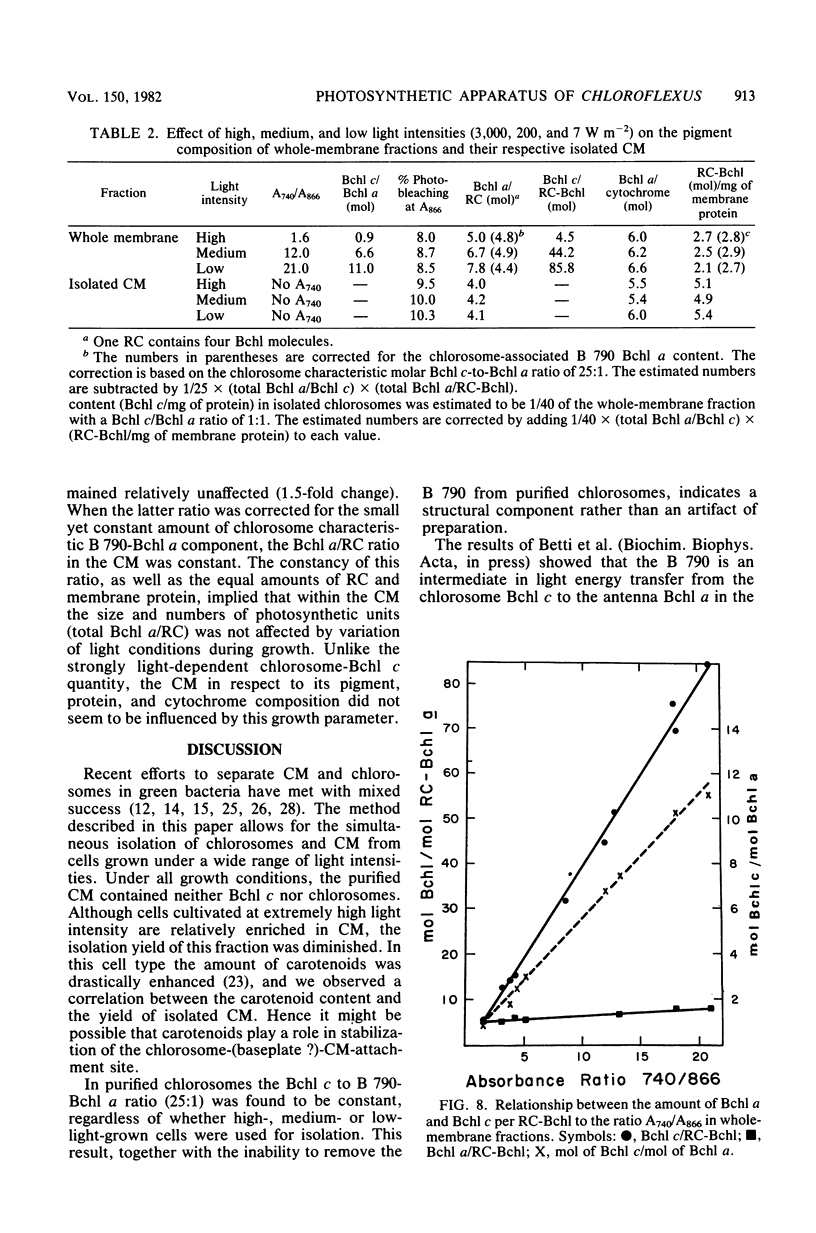


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aagaard J., Sistrom W. R. Control of synthesis of reaction center bacteriochlorophyll in photosynthetic bacteria. Photochem Photobiol. 1972 Feb;15(2):209–225. doi: 10.1111/j.1751-1097.1972.tb06240.x. [DOI] [PubMed] [Google Scholar]
- Broch-Due M., Ormerod J. G., Fjerdingen B. S. Effect of light intensity of vesicle formation in chlorobium. Arch Microbiol. 1978 Mar;116(3):269–274. doi: 10.1007/BF00417850. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., PFENNIG N., KUNISAWA R. THE FINE STRUCTURE OF GREEN BACTERIA. J Cell Biol. 1964 Jul;22:207–225. doi: 10.1083/jcb.22.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. K., Straley S. C. Photochemical electron transport in photosynthetic reaction centers. IV. Observations related to the reduced photoproducts. Biophys J. 1972 Oct;12(10):1221–1234. doi: 10.1016/S0006-3495(72)86158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. L., Niederman R. A. Membranes of Rhodospirillum rubrum: isolation and physicochemical properties of membranes from aerobically grown cells. J Bacteriol. 1976 Jun;126(3):1316–1325. doi: 10.1128/jb.126.3.1316-1325.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruden D. L., Stanier R. Y. The characterization of chlorobium vesicles and membranes isolated from green bacteria. Arch Mikrobiol. 1970;72(2):115–134. doi: 10.1007/BF00409518. [DOI] [PubMed] [Google Scholar]
- Ding D. H., Kaplan S. Separation of inner and outer membranes of Rhodopseudomonas spheroides. Prep Biochem. 1976;6(1):61–79. doi: 10.1080/00327487608061599. [DOI] [PubMed] [Google Scholar]
- Fowler C. F., Nugent N. A., Fuller R. C. The isolation and characterization of a photochemically active complex from Chloropseudomonas ethylica. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2278–2282. doi: 10.1073/pnas.68.9.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloe A., Risch N. Bacteriochlorophyll cs, a new bacteriochlorophyll from Chloroflexus aurantiacus. Arch Microbiol. 1978 Aug 1;118(2):153–156. doi: 10.1007/BF00415723. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Conti S. F., Fuller R. C. Effect of light intensity on the formation of the photochemical apparatus in the green bacterium Chloropseudomonas ethylicum. J Bacteriol. 1966 Jan;91(1):349–355. doi: 10.1128/jb.91.1.349-355.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Conti S. F., Fuller R. C. Photosynthetic Apparatus in the Green Bacterium Chloropseudomonas ethylicum. J Bacteriol. 1966 Jan;91(1):311–323. doi: 10.1128/jb.91.1.311-323.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. T., Drews G. The respiratory electron transport system of heterotrophically-grown Rhodopseudomonas palustris. Arch Microbiol. 1975 Mar 10;102(3):219–231. doi: 10.1007/BF00428372. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanne B., Vänngård T. Redox titrations of cytochrome c oxidase. An analysis of a multi-electron system. Biochim Biophys Acta. 1978 Mar 13;501(3):449–457. doi: 10.1016/0005-2728(78)90112-3. [DOI] [PubMed] [Google Scholar]
- Olson J. M., Giddings T. H., Jr, Shaw E. K. An enriched reaction center preparation from green photosynthetic bacteria. Biochim Biophys Acta. 1976 Nov 9;449(2):197–208. doi: 10.1016/0005-2728(76)90133-x. [DOI] [PubMed] [Google Scholar]
- Olson J. M., Shaw E. K., Englberger F. M. Comparison of bacteriochlorophyll a-proteins from two green bacteria. Biochem J. 1976 Dec 1;159(3):769–774. doi: 10.1042/bj1590769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson B. K., Castenholz R. W. A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch Microbiol. 1974;100(1):5–24. doi: 10.1007/BF00446302. [DOI] [PubMed] [Google Scholar]
- STANIER R. Y., SMITH J. H. The chlorophylis of green bacteria. Biochim Biophys Acta. 1960 Jul 15;41:478–484. doi: 10.1016/0006-3002(60)90045-7. [DOI] [PubMed] [Google Scholar]
- SYBESMA C., OLSON J. M. Transfer of chlorophyll excitation energy in green photosynthetic bacteria. Proc Natl Acad Sci U S A. 1963 Feb 15;49:248–253. doi: 10.1073/pnas.49.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague S. G., Staehelin L. A., DiBartolomeis M. J., Fuller R. C. Isolation and development of chlorosomes in the green bacterium Chloroflexus aurantiacus. J Bacteriol. 1981 Sep;147(3):1021–1031. doi: 10.1128/jb.147.3.1021-1031.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague S. G., Staehelin L. A., Fuller R. C. Semiaerobic induction of bacteriochlorophyll synthesis in the green bacterium Chloroflexus aurantiacus. J Bacteriol. 1981 Sep;147(3):1032–1039. doi: 10.1128/jb.147.3.1032-1039.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A., Golecki J. R., Drews G. Supramolecular organization of chlorosomes (chlorobium vesicles) and of their membrane attachment sites in Chlorobium limicola. Biochim Biophys Acta. 1980 Jan 4;589(1):30–45. doi: 10.1016/0005-2728(80)90130-9. [DOI] [PubMed] [Google Scholar]
- Straley S. C., Parson W. W., Mauzerall D. C., Clayton R. K. Pigment content and molar extinction coefficients of photochemical reaction centers from Rhodopseudomonas spheroides. Biochim Biophys Acta. 1973 Jun 28;305(3):597–609. doi: 10.1016/0005-2728(73)90079-0. [DOI] [PubMed] [Google Scholar]
- Swarthoff T., Amesz J. Photochemically active pigment-protein complexes from the green photosynthetic bacterium Prosthecochloris aestuarii. Biochim Biophys Acta. 1979 Nov 8;548(2):427–432. doi: 10.1016/0005-2728(79)90146-4. [DOI] [PubMed] [Google Scholar]
- Swarthoff T., van der Veek-Horsley K. M., Amesz J. The primary charge separation, cytochrome oxidation and triplet formation in preparations from the green photosynthetic bacterium Prosthecochloris aestuarii. Biochim Biophys Acta. 1981 Mar 12;635(1):1–12. doi: 10.1016/0005-2728(81)90002-5. [DOI] [PubMed] [Google Scholar]
- Van der Rest M., Gingras G. The pigment complement of the photosynthetic reaction center isolated from Rhodospirillum rubrum. J Biol Chem. 1974 Oct 25;249(20):6446–6453. [PubMed] [Google Scholar]