Abstract
The interleukin-2 (IL-2)/IL-2 receptor (IL-2R) system is the main regulatory determinant of T cell reactivity. Although it is well known that IL-2 secretion is impaired during HIV infection, up to now IL-2R expression has not been extensively studied in HIV-infected patients despite the use of IL-2 in clinical therapy trials. We show here that IL-2R expression in HIV patients with high viral load (group 1 in the study) is greatly enhanced on B lymphocytes, CD8 T lymphocytes, and monocytes, but not on CD4 T lymphocytes, compared with noninfected individuals. Paradoxically, this modified IL-2R expression does not lead to increased IL-2 responsiveness, except for B lymphocytes. In patients receiving triple combination therapy (TCT, two reverse transcriptase inhibitors and one protease inhibitor) that has triggered a drastic reduction in plasma viral load and an increase in CD4 counts (group 2 patients), IL-2R expression is significantly lower than in group 1 patients. Moreover, cells involved in cellular immunity and CD4 T lymphocytes have the capacity to respond to IL-2 after TCT. These results allow us to anticipate a beneficial role of IL-2 immunotherapy in combination with TCT.
The primary role of interleukin-2 (IL-2) is to expand the population of activated T lymphocytes (1). We have previously shown that IL-2 is the main cytokine controlling the proliferation of anti-CD3-stimulated CD4 T lymphocytes (2). IL-2 also stimulates the cytotoxic activity of CD8 T lymphocytes and NK cells, proliferation and Ig production by activated B lymphocytes, and some monocyte functions (for review see refs. 3–7). The IL-2 receptor (IL-2R) is made up of three transmembrane proteins; in humans two forms of the IL-2R are functional. The intermediate-affinity IL-2R (Kd = 1 nM) is formed by the IL-2Rβ and IL-2Rγ chains, whereas the complex IL-2Rα, IL-2Rβ, and IL-2Rγ constitutes the high-affinity receptor (Kd = 10 pM). IL-2Rβ and IL-2Rγ chains are shared by receptors of other cytokines (for review, see refs. 8–11).
Decreases in IL-2 production and IL-2Rα expression during HIV infection have been reported (12). However, the pattern of expression of IL-2Rβ and IL-2Rγ (the most critical components involved in IL-2 signal transduction) has not been well studied, the data concerning IL-2Rβ are contradictory (13–15), and there is no information concerning IL-2Rγ in this pathology. Here we analyze the expression of the three IL-2R chains by CD4 T, CD8 T, B, and natural killer (NK) lymphocytes, as well as monocytes, of HIV-infected patients with high viral load. In HIV-infected patients, IL-2R expression clearly differs from the pattern that we have previously established for healthy donors (16).
Recently, new antiretroviral therapies combining two inhibitors of HIV reverse transcriptase (RT) and one inhibitor of HIV protease, usually called triple combination therapy (TCT) or highly active antiretroviral therapy (HAART), have been successful in leading to a significant reduction of plasmatic viral load and an increase in CD4 counts (17, 18). The functionality of the immune system of patients receiving this potent antiretroviral combination is still a matter of intensive investigation. Preliminary results indicate that TCT has positive effects on some functions of CD4 T lymphocytes (18), but the properties of other peripheral blood mononuclear cell (PBMC) subsets have not been well studied. Here, we study the pattern of IL-2Rα, IL-2Rβ, and IL-2Rγ expression and IL-2 responsiveness of the different PBMC subsets from patients undergoing TCT. We show that the pattern of IL-2R expression is close to normal and that under TCT several PBMC subsets display a clear IL-2 reactivity. Our results have strong implications for the possible association of TCT and IL-2 in future HIV therapies because IL-2 is known to enhance the level of CD4 T cells in HIV-infected patients (19, 20).
MATERIALS AND METHODS
Patients.
Two groups of HIV-infected patients (followed at Hôpital de l’Institut Pasteur) were studied.
The first group (group 1) comprised 21 patients with high viral load: 2 × 105 copies per ml at the mean (range: 3 × 103 to 7 × 105) (Amplicor Roche assay). The CD4 count mean of this group was 303 per mm3 (range: 14–924). All these patients received one or two RT inhibitors at usual dosage. Ten received one RT inhibitor: 3′-azido-3′-deoxythymidine (AZT), n = 5; 2′,3′-dideoxyinosine (DDI), n = 4; 2′,3′-didehydro-3′-deoxythymidine (D4T), n = 1. Eleven patients received two RT inhibitors: DDI + 2′,3′-dideoxy-3′-thiacytidine (3TC), n = 3; AZT + 2′,3′-dideoxycytidine (DDC), n = 2; AZT + DDI, n = 2; AZT + 3TC, n = 2; 3TC + D4T: n = 2.
The second group of patients (group 2, n = 27) received TCT. The drug regimens of this group were as follows: 3TC + D4T + indinavir, n = 13; 3TC + AZT + indinavir, n = 6; 3TC + D4T + ritonavir, n = 5; 3TC + AZT + ritonavir, n = 1; 3TC + D4T + saquinavir, n = 1; 3TC + AZT + saquinavir, n = 1. Mean TCT duration was 7 months (1–15) at the time of blood sampling. TCT triggered a drastic decrease in viral load, which became undetectable (<200 copies per ml) in 18 of 27 cases, or very low for the other 9 patients (<1,000 copies per ml). Initial viral burden of these patients was 3 × 105 at the mean (range: 2 × 104 to 2 × 106). CD4 counts per mm3 increased from 94 (5–343) to 310 (92–735).
Characteristics of patients in groups 1 and 2 were otherwise comparable: mean age, 40 years (range, 22–67); gender, 7/48 female, 41/48 male; mean HIV-infection length, 7 years (range, 1–14); risk factors, 31/48 homo- or bisexual, 7/48 heterosexual, 6/48 intravenous drug users 2/48 blood recipients, 2/48 unknown. CD4 counts were comparable in the two groups of patients at the time the blood samples were taken.
Healthy donors (Centre de Transfusion Sanguine “Jean Julliard”) were used as controls during the course of our investigation.
Blood Collection and PBMC Preparation.
Venous blood from healthy donors and HIV-infected patients was typically collected with sodium heparin as anticoagulant. On the day the blood sample was collected, PBMC were isolated by Ficoll/Hypaque (Pharmacia) sedimentation and were immediately stained and analyzed with flow cytometry or stimulated by IL-2 (see below).
Antibodies and Reagents.
mAbs anti-IL-2Rα (33B3, IgG2a) and anti-IL-2Rβ (CF1, IgG1) were obtained from Immunotech (Luminy, France). The properties of IgG mAb anti-IL-2Rγ (3B5) have been described (21).
Mouse mAbs, isotype-matched controls, and Fab fragments used to discriminate the different PBMC subsets were the same as in our previous study (16). Anti-CD69 (TP1.55.3, IgG1) and anti-CD71 (Ber-T9, IgG1) were obtained from Immunotech and Dako, respectively. These antibodies were used at saturating concentrations.
Flow Cytometric Analysis of IL-2R Expression.
Staining, acquisition, and analysis were performed as described (16). Briefly, PBMC were first incubated with mAbs for IL-2Rα, IL-2Rβ, or IL-2Rγ components or for activation markers (CD69, CD71). After washing, cells were incubated with fluorescein isothiocyanate (FITC)-conjugated Fab fragment goat anti-mouse IgG (H + L chains). Cells were finally incubated with CD4-PE + CD14-TC, CD20-PE + CD14-TC, CD56-PE + CD14-TC, or CD8-PE mAb (PE, phycoerythrin; TC, Tri color). A total of 2 × 104 PBMC per sample were acquired for CD4, CD56, and CD8 cells and of 5 × 104 for CD20 cells. Data acquisition and analysis were performed with a FACScan flow cytometer using cellquest 3.1 software (Becton Dickinson). Specific immunofluorescence was discriminated from background by staining with isotype-matched control mAbs. CD14-TC staining was used to exclude monocytes by gating at the time of analysis.
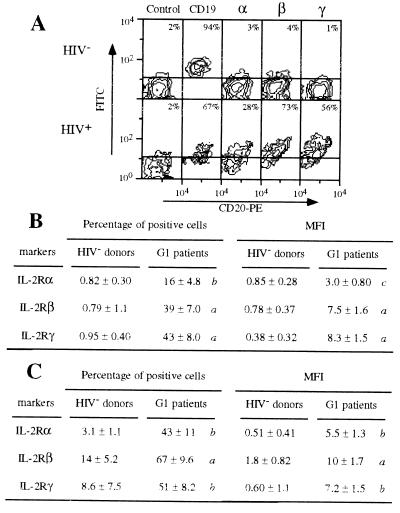
Results for IL-2R expression are indicated for each PBMC subset as the percentage of positive cells on the FL1 scale—i.e., the proportion of cells with fluorescence higher than background. Background is defined as the fluorescence obtained by staining with isotype-matched Ab. The threshold value is defined by the cells with the brightest background. When positive cells are found, the entire population is shifted and the mean fluorescence intensity (MFI) increases. Thus, a higher percentage of positive cells always correlates with a higher MFI (see Fig. 1 B and C).
Figure 1.
Expression of IL-2Rα, IL-2Rβ, and IL-2Rγ by B and CD8 T lymphocytes of group 1 patients (high viral load). (A) IL-2R expression by B lymphocytes of a representative healthy donor (HIV−) and of a representative group 1 HIV-infected patient (HIV+). PBMC were treated by mAbs 33B3 (anti-IL-2Rα), CF1 (anti-IL-2Rβ), 3B5 (anti-IL-2Rγ), and isotype-matched controls. FITC-labeled Fab fragment anti-IgG was used, followed by PE-conjugated anti-CD20 + TC-conjugated anti-CD14 mAbs. Only the two FL2-positive squares—i.e., CD20-positive cells—are shown. The percentage of B lymphocytes located above (FL1 scale) the quadrant settings is indicated. Markers used for FL1 scale are shown on the top. (B) Expression of IL-2R chains by B lymphocytes from healthy donors (HIV− donors, n = 14) and from group 1 patients (G1 patients, n = 10). Expression is indicated by the mean percent (±SD) of B lymphocytes positive for the IL-2R chains and by the MFI (±SD) of the B lymphocytes. P values (nonpaired Student’s t test) are shown as follows: a, P ≤ 0.0001; b, 0.0001 < P ≤ 0.001; c, 0.001 < P ≤ 0.01; and d, 0.01 < P < 0.05. P values of ≥ 0.05 are not shown (indicated as NS in Table 1). (C) Expression of IL-2R chains by CD8 T lymphocytes from healthy donors (HIV− donors, n = 12) and from group 1 patients (G1 patients, n = 10). Expression is indicated by the mean percent (±SD) positive cells and by the MFI (±SD) of the population. P values are indicated as in B.
Cell Cycle Analysis.
DNA was stained with propidium iodide in freshly isolated PBMC and in PBMC harvested after one night in 5% fetal calf serum/RPMI 1640 medium (106 cells per ml) both in the presence and in the absence of recombinant IL-2 (5 × 10−8 M; Chiron). Where indicated, cells were stimulated with phytohemagglutinin (PHA; 5 μg/ml). Cell-surface labeling was first performed to distinguish between different PBMC subsets. Cells were incubated with mAbs for CD4, CD19 + CD20, CD16 + CD56, or CD8, washed, and incubated with FITC-conjugated Fab fragment goat anti-mouse IgG (H + L) as described above. Cells were then fixed and permeabilized as described (2), and incubated with a propidium iodide (50 μg/ml) and RNase (25 μg/ml) solution for 30 min at room temperature. A total of 105 cells was acquired. Cell doublets were excluded on FL2-width/FL2-area dot-plots. The studied PBMC subset was selected by gating FL1 positive cells. Events were plotted in a one-parameter histogram on a linear scale. The percentage of cells in each region (G0/G1, S, G2/M) was determined with the modfit lt 2.0 program (Becton Dickinson). Results are expressed as percentage of cells in S+G2/M due to IL-2 stimulation (% of cells in S+G2/M at day 1 with IL-2 − % of cells in S+G2/M at day 1 without IL-2). PHA response is considered as a maximum positive response.
RESULTS
Overexpression of the Three IL-2R Components on B and CD8 T Lymphocytes of HIV-Infected Patients with High Viral Load (Group 1).
This group of patients received one or two RT inhibitors but not protease inhibitor. Their plasmatic viral load was 2 × 105 copies per ml on the average. The results were compared with data obtained from a group of healthy donors.
The three IL-2R chains are clearly expressed at the cell surface of B lymphocytes from these patients. Fig. 1A shows the results obtained with a typical patient. The mean number of positive cells is 16%, 39%, and 43% for IL-2Rα, IL-2Rβ, and IL-2Rγ, respectively, whereas these chains are undetectable on cells from healthy donors (Fig. 1B). The higher expression of the three IL-2R components on B lymphocytes is also seen when MFI of these cells from normal individuals and patients are compared (Fig. 1B). CD69 activation marker is more expressed at the surface of B lymphocytes from group 1 patients, compared with healthy donors: 13% ± 5.1% vs. 1.8% ± 0.39% (P < 0.003). A similar pattern is observed for the CD71 activation marker expression: 11% ± 2.5% vs. 2.3% ± 0.48% (P < 0.0004).
A higher expression of IL-2Rα, IL-2Rβ, and IL-2Rγ is also detected on CD8 T lymphocytes of group 1 patients, compared with healthy donors (Fig. 1C). The results correlate when the data are expressed as a percentage of positive cells or in terms of MFI. Mean percentages of IL-2R-positive cells are even greater for CD8 T lymphocytes than for B lymphocytes: 43%, 67%, and 51% for IL-2Rα, IL-2Rβ, and IL-2Rγ, respectively (Fig. 1C). Surprisingly, CD69 and CD71 expression at the surface of CD8 T lymphocytes is similar both in patients and in control individuals (data not shown).
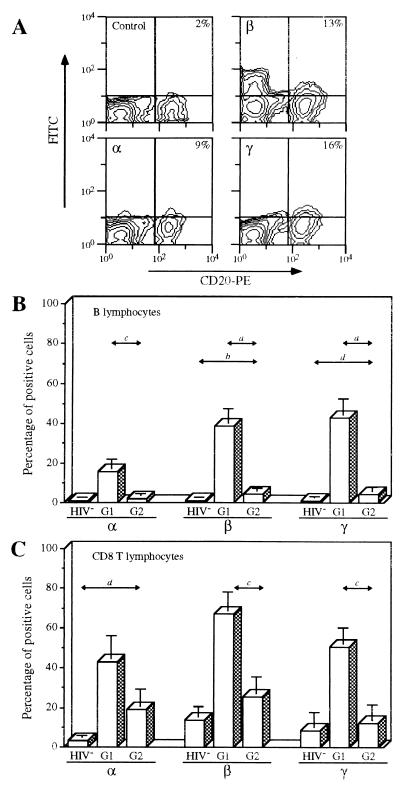
Different IL-2R Expression by B and CD8 T Lymphocytes of Patients under TCT/HAART (Group 2).
Expression of the three IL-2R chains by the PBMC subsets from patients receiving TCT (group 2) was examined. TCT therapy triggered an increase in CD4 counts and a decrease in viral load.
IL-2Rα, IL-2Rβ, and IL-2Rγ were undetectable or barely present on B lymphocytes from group 2 patients, and less expressed than in patients of group 1 (Fig. 2 A and B). The pattern of IL-2R expression is comparable to that obtained with cells of healthy donors (mean number of positive cells in the group < 5%). A similar lower expression is observed for CD69 and CD71 expression markers: 3.6% ± 1.3% and 5.9% ± 1.3%, respectively.
Figure 2.
Expression of IL-2Rα, IL-2Rβ, and IL-2Rγ by B and CD8 T lymphocytes of HIV-infected patients treated with TCT (group 2); labeling and analysis of the cells were performed as described in the legend of Fig. 1. (A) IL-2R expression by B lymphocytes of a representative patient receiving TCT (group 2). (B) Mean expression (±SD) of IL-2R by B lymphocytes of the three groups of individuals: healthy donors (HIV−, n = 14), group 1 patients (G1, n = 10), and group 2 (TCT) patients (G2, n = 12). P values are indicated as in Fig. 1. (C) Mean expression (±SD) of IL-2R by CD8 lymphocytes of the three groups of individuals: healthy donors (HIV−, n = 12), group 1 patients (G1, n = 10), and group 2 patients (G2, n = 12). P values are indicated as in Fig. 1.
For CD8 T lymphocytes, IL-2R expression is significantly lowered for patients undergoing TCT, compared with group 1 patients: IL-2Rα, IL-2Rβ, and IL-2Rγ expression is 19%, 26%, and 12%, respectively (Fig. 2C). Despite the fact that IL-2Rβ and IL-2Rγ expression is not abolished, the mean expression is not significantly different from that of healthy donors. Within this subset, CD69 and CD71 expression is similar in group 2 and group 1 patients (data not shown).
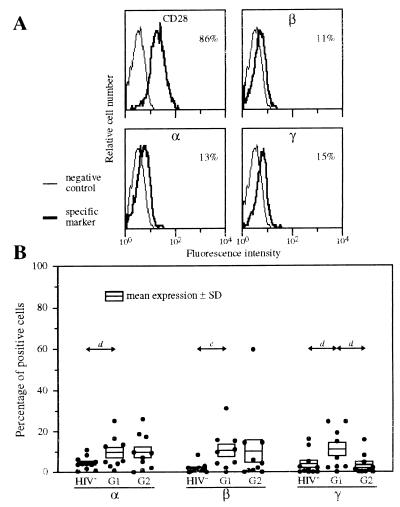
Low IL-2R Expression by CD4 T Lymphocytes of Patients with High Viral Load (Group 1) and TCT Patients with Very Low Viral Load (Group 2).
Although IL-2R chains are highly expressed on B and CD8 T lymphocytes of group 1 patients (see Fig. 1), their expression is low on CD4 T lymphocytes: 9.8%, 11%, and 11% of positive cells for IL-2Rα, IL-2Rβ, and IL-2Rγ, respectively (Fig. 3). However, this expression is slightly higher than normal. CD69 and CD71 expression remains similar to normal (data not shown).
Figure 3.
Expression of IL-2Rα, IL-2Rβ, and IL-2Rγ by CD4 T lymphocytes of HIV-infected patients. PBMC were treated by mAbs 33B3, CF1, 3B5, or IOT28 (anti-CD28). Characterization of the population was achieved by treatment with anti-CD3 mAb (data not shown). FITC-labeled Fab fragment anti-IgG was used, followed by PE-conjugated anti-CD4 + TC-conjugated anti-CD14 mAbs. (A) IL-2R expression of a representative group 1 patient. The percentage of CD4 T lymphocytes positive for the different IL-2R chains is indicated. (B) Expression of IL-2Rα, IL-2Rβ, and IL-2Rγ by CD4 T lymphocytes of healthy donors (HIV−, n = 11), group 1 patients (G1, n = 9), and group 2 (TCT) patients (G2, n = 10). Mean numbers of positive cells (±SD) are shown. P values are indicated as in Fig. 1.
CD4 T lymphocytes of TCT patients (group 2) also express IL-2R chains at low levels: ≤10% (Fig. 3B). The results are similar (except for IL-2Rγ) to those obtained with group 1 patients. CD71 expression is higher on cells of group 2 patients: 12% ± 2.8%, versus 6.3% ± 1.1% (P < 0.04) for group 1 patients. Whereas CD28 expression is lower than normal in group 1 patients: 74% ± 9.1% vs. 95% ± 0.92% (P < 0.02), the number of CD28-positive CD4 T lymphocytes is higher under TCT: 92% ± 2.1% (P < 0.03), and expression is quite similar to normal.
Expression of the Three IL-2R Components by Monocytes and NK Cells of Group 1 and Group 2 Patients.
IL-2Rγ is more highly expressed on monocytes of patients with high viral load (group 1), compared with healthy donors (78% vs. 49%, Table 1). Furthermore, IL-2Rβ is expressed on monocytes of group 1 patients (22% of positive cells), whereas it is almost undetectable for healthy donors (Table 1). On the other hand, expression of IL-2Rβ and IL-2Rγ is lower on monocytes from group 2 patients, compared with group 1, and the pattern of IL-2R expression is close to normal under TCT (Table 1). For CD16, CD69, and CD71 activation markers, a similar pattern is observed: higher expression for group 1 patients, compared with healthy donors and lower expression for TCT patients (group 2), compared with group 1 patients (data not shown). Monocyte expression of IL-2Rα remains unchanged among the three groups of individuals (Table 1).
Table 1.
Expression of IL-2R by monocytes and NK cells
| IL-2R chain | Percentage of positive cells
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Monocytes
|
NK cells
|
|||||||||
| Healthy donors (n = 15) | Group 1 patients (n = 10) | P | Group 2 patients (n = 10) | P | Healthy donors (n = 13) | Group 1 patients (n = 10) | P | Group 2 patients (n = 8) | P | |
| α | 2.4 ± 0.69 | 4.7 ± 1.2 | d* | 3.1 ± 1.0 | NS§ | 1.4 ± 0.32 | 5.6 ± 2.4 | d* | 2.5 ± 0.92 | NS§ |
| β | 5.1 ± 1.2 | 22 ± 6.3 | c* | 5.7 ± 2.2 | d§ | 78 ± 3.1 | 71 ± 6.5 | NS* | 77 ± 4.8 | NS§ |
| γ | 49 ± 6.0 | 78 ± 5.9 | c* | 36 ± 3.4 | a§ | 3.0 ± 0.46 | 6.6 ± 2.8 | NS* | 2.7 ± 0.83 | NS§ |
Expression of IL-2R chains by monocytes was determined as indicated in Fig. 1 and by gating CD14+–CD4low cells; expression of IL-2R chains by NK cells was determined as explained in Materials and Methods, and by gating CD56+–CD14− cells. P values are indicated as in Fig. 1; NS, not significant. ∗, Compared to healthy donors;
, compared to group 1 patients. In all cases, there are no significant differences between values of healthy donors and values of group 2 patients.
For NK cells, no difference in the expression of the three IL-2R components is observed among the two groups of HIV-infected patients and the group of healthy donors (Table 1). IL-2Rα expression is slightly higher in group 1 patients (high viral load), compared with healthy donors, but its expression remains marginal (<5% of positive cells).
Comparison of the IL-2 Response of PBMC Subsets from Healthy Donors, Group 1 Patients, and Group 2 Patients.
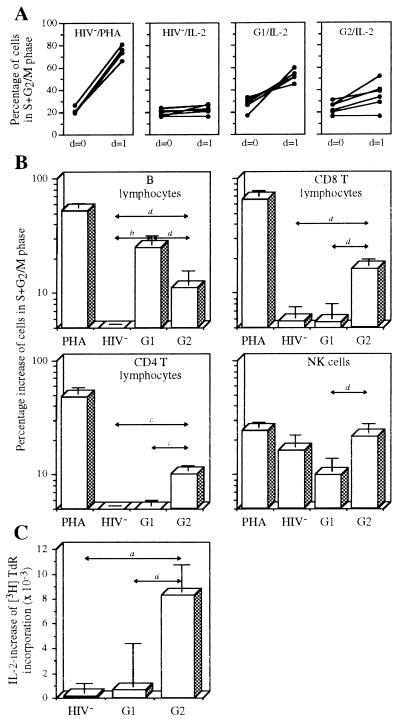
IL-2 response of PBMC subsets was analyzed in vitro by entry of lymphocytes into the cell cycle after IL-2 stimulation. The percentage of cells in S+G2/M phase at day 0 and day 1 after IL-2 stimulation were compared.
B lymphocytes behave as expected by the level of IL-2R expression (Fig. 4 A and B). Indeed, <5% of B cells from healthy donors respond to IL-2, whereas IL-2 responsiveness of group 1 (high viral load) patients is higher (25%). Under TCT (group 2), B cell IL-2 response is lower.
Figure 4.
IL-2 response of the different PBMC subsets. (A) Response of B lymphocytes. Cells from healthy donors were stimulated by PHA (HIV−/PHA) or IL-2 (HIV−/IL-2). Cells from group 1 patients (G1/IL-2) and from group 2 (TCT) patients (G2/IL-2) were stimulated by IL-2. Percentage of cells in S+G2/M phase before (day 0) and after (day 1) stimulation are shown for each individual. (B) Responses of B lymphocytes, CD8 T lymphocytes, CD4 T lymphocytes, and NK cells. The four conditions shown in A were studied. Mean percentage of cells in S+G2/M phase after overnight stimulation with PHA or IL-2 are shown (HIV−, n = 7; G1, n = 10; G2, n = 10). Background obtained with unstimulated cells is removed. P values are indicated as in Fig. 1. (C) PBMC proliferation. PBMC (5 104 per well) were cultured in 96-well flat-bottom plates in a total volume of 200 μl of RPMI 1640 medium with PHA (5 μg/ml) and PHA + IL-2 (5 × 10−8 M). After 2 days, cultures were pulsed for 18 h with 1 μCi (1 Ci = 37 GBq) of [3H]thymidine, and incorporated radioactivity was measured. Variation of incorporated radioactivity between PHA+IL-2 and PHA alone is shown for healthy donors (HIV−, n = 18), group 1 patients (G1, n = 6), and group 2 patients (G2, n = 5). P values are indicated as in Fig. 1.
Although CD8 T lymphocytes of group 1 patients highly express the three IL-2R chains (Fig. 1), IL-2 has only a marginal effect on their entry into the cell cycle (Fig. 4B). Interestingly, although the level of IL-2R expression is lower on CD8 T lymphocytes from group 2 patients (Fig. 3C), these lymphocytes are clearly reactive to IL-2.
CD4 and CD8 T lymphocytes show a quite similar pattern of IL-2 responsiveness: cells of healthy donors and of group 1 patients show only a marginal IL-2 response (Fig. 4B). It is noteworthy that CD4 T lymphocytes are able to respond to IL-2 for the patients who have received TCT (group 2).
NK cells of healthy donors clearly respond to IL-2. When the IL-2 reactivity of NK cells from group 1 patients and from group 2 patients was compared, a significantly higher IL-2 reactivity is found for patients undergoing TCT (Fig. 4B).
IL-2 sensitivity of PHA-stimulated PBMC from healthy donors, group 1 patients, and group 2 patients was compared. In agreement with the data reported above, PHA-blasts from TCT patients were much more sensitive to IL-2 than were the corresponding cells from group 1 patients (Fig. 4C).
DISCUSSION
We have studied expression of the three IL-2R chains and the IL-2 reactivity of the five major PBMC subsets in two groups of HIV-infected patients. The main data are summarized in Table 2. The first group has high viral load, and patients were treated with one or two RT inhibitors. The second group has an undetectable or a very low viral burden and received TCT associating two RT inhibitors and one protease inhibitor. On the average, both groups have comparable CD4 counts. For the second group, the CD4 counts had increased by a factor of 3 in the average under the treatment. The data summarized in Table 2 suggest that there is a dysregulation of the IL-2/IL-2R system during HIV infection: (i) expression of the three chains of the IL-2R is higher in B and CD8 T lymphocytes of HIV-infected patients with high viral load (group 1) compared with healthy donors; (ii) the strong expression of the IL-2R by CD8 T lymphocytes of group 1 patients without appearance of IL-2 reactivity also suggests an influence of HIV-infection on the functionality of the IL-2R; (iii) this strong overexpression is not seen in the group of patients receiving TCT with an undetectable or a very low viral load (group 2). These abnormalities seem to be specific to HIV infection, because preliminary data obtained with PBMC from patients chronically infected with hepatitis B virus (HBV) do not show IL-2R dysregulation (D.D., unpublished data). Table 2 also reports the impact of TCT/HAART in the IL-2 reactivity of HIV-infected patients (see below for discussion).
Table 2.
IL-2R expression and IL-2 reactivity of B, CD8 T, and CD4 T lymphocytes from group 1 and group 2 patients
| Healthy donors | Group 1 patients | Group 2 patients | |
|---|---|---|---|
| Clinical characteristics | |||
| Viral load, copies per ml | 2 × 105 | <200* | |
| CD4 T counts per mm3 | 303 | 310† | |
| Treatment | 1 or 2 RT inhibitors | 2 RT inhibitors + 1 protease inhibitor | |
| B lymphocytes | |||
| IL-2R expression‡ | 0.8/0.8/0.9 | 16/39/43 | 2/4/4 |
| IL-2 reactivity§ | 3 | 25 | 11 |
| CD8 T lymphocytes | |||
| IL-2R expression‡ | 3/14/9 | 43/67/51 | 19/26/12 |
| IL-2 reactivity§ | 6 | 6 | 16 |
| CD4 T lymphocytes | |||
| IL-2R expression‡ | 5/2/4 | 10/11/11 | 10/10/3 |
| IL-2 reactivity§ | 4 | 4 | 10 |
In 18 of 27 patients viral burden became undetectable (<200 copies per ml) and in 9 of 27 patients decreased at <1,000 copies/ml.
Initial CD4 T count mean of group 1 patients was 94 per mm3 before TCT.
Mean percent of positive cells for IL-2Rα/IL-2Rβ/IL-2Rγ, respectively.
Mean percent of cells entering in S + G2/M phase after in vitro IL-2 stimulation.
It is particularly interesting to compare the properties of B and CD8 T lymphocytes of HIV-infected patients with high viral load (group 1). Whereas B lymphocytes of healthy donors are at a resting stage—i.e., IL-2R expression and IL-2 response are undetectable—these cells are activated during HIV infection. Indeed, they express high amounts of the three IL-2R components (Fig. 1) as well as the CD69 and CD71 activation markers (see Results). The high IL-2R expression may explain their strong IL-2 responsiveness (Fig. 4) and may also trigger sensitivity to cytokines such as IL-4, IL-7, or IL-15, which use IL-2Rβ and/or IL-2Rγ for signal transduction. This result is consistent with the overactivity of B lymphocytes in HIV infection (reviewed in ref. 22). For CD8 T lymphocytes of this group of patients, the IL-2/IL-2R system is even more dysregulated. Although these cells do not overexpress CD69 and CD71 activation markers, they show strong IL-2R expression but do not respond to IL-2 (Figs. 1 and 4). This result is in agreement with the underexpression of STAT 5 in PBMC of HIV-infected patients (23). We have previously shown that high IL-2R expression does not always lead to IL-2 responsiveness when IL-2 was lacking during the activation step (5), as in the case in HIV-infected patients (12, 24). These observations may explain, in part, the final lack of effectiveness of the cytotoxic T lymphocytic response elicited by HIV infection (25).
TCT leads to a strong decrease in plasmatic viral load and an increase in CD4 T cell counts. However, the functionality of the newly circulating CD4 T lymphocytes is still under investigation and the characteristics of the other PBMC subsets have been poorly determined. Here we show that the pattern of IL-2R expression is clearly different under TCT (group 2): B lymphocytes, CD8 T lymphocytes, and monocytes more weakly express IL-2R chains compared with group 1, and the pattern is close to normal (Fig. 2, Table 1). For B lymphocytes, there is also a lower IL-2 responsiveness (Fig. 4), although this response is higher than in cells of healthy donors. There is therefore a lower overactivity of B lymphocytes under TCT, although cells are not in the resting stage found in healthy donors. In our studies, B lymphocytes may be considered as control cells because their pattern of IL-2 reactivity clearly follows expression of IL-2R. For CD8 T lymphocytes, IL-2R expression is lower under TCT, as if their overactivity were diminished. Furthermore, during TCT, CD8 T lymphocytes have the capacity to respond to IL-2 (Fig. 4). Concomitantly, NK cells of TCT patients also display higher IL-2 responses (Fig. 4). One may conclude from these observations that responses of the cellular/cytotoxic compartment are potentially enhanced by TCT.
In contrast to B and CD8 T lymphocytes, IL-2R expression is only slightly higher on CD4 T lymphocytes of group 1 patients (high viral load), compared with cells of healthy donors (Fig. 3). Accordingly, the IL-2 response of these cells is marginal, as for cells of healthy donors. This low IL-2R expression and low IL-2 response are consistent with the hyporeactivity of CD4 T lymphocytes of HIV-infected patients. Indeed, it was previously shown that these cells are not responsive to various stimuli, and the response is not improved upon addition of exogenous IL-2 (26–28). Lack of IL-2 may be responsible for the anergic state of the CD4 T lymphocytes (29) and for their inability to activate IL-2R gene expression. Alternatively, the low IL-2R expression on CD4 T lymphocytes may be caused by free HIV glycoprotein gp120. Indeed, we have previously shown that gp120 impedes IL-2R expression on CD4 T lymphocytes of healthy donors upon in vitro stimulation, and that residual IL-2Rs are not functional (30). Whereas CD4 T lymphocytes of group 1 patients only marginally respond to IL-2, this subset is clearly able to respond to IL-2 for patients with TCT/HAART (group 2, Fig. 4). This IL-2-responsive state, as well as high expression of CD71 (see Results) and HLA-DR (data not shown), indicates that these cells are not in a resting stage like cells of healthy donors. IL-2 responsiveness of CD4 T lymphocytes from TCT patients is consistent with a higher expression of CD28 on these cells, compared with group 1 patients (see Results). As previously shown by Autran et al. (18), we also found that CD4 T lymphocytes of TCT patients are mainly composed of CD45RO memory cells (data not shown). Our data suggest that newly circulating CD4 T lymphocytes show some characteristics of cells functionally able to participate in immune functions.
In our study, a reduction in the overactivity of the immune system, particularly of cells involved in the humoral response, is observed under TCT/HAART. This extends the results of Autran et al. (18) showing a decrease in CD38 expression on T lymphocytes. The reduction in the overactivity of the immune system is certainly beneficial, as this phenomenon has been linked to progression to AIDS and to several negative effects such as sensitivity to apoptosis (31–33). However, the immune system of TCT patients is not at resting stage as in the case of healthy donors; indeed, IL-2R and activation markers are often more expressed in TCT patients than in healthy donors. Furthermore, cells of the cellular immunity compartment, CD8 T lymphocytes and NK cells, and CD4 T lymphocytes are sensitive to IL-2 under TCT, whereas the same PBMC subsets of healthy donors are not. These features may reflect an active stage of the immune system due to a persistent viral infection, as suggested by recent results showing an HIV reservoir in TCT patients (34–36). It would be of interest to determine if these cells are able to participate in virus elimination after TCT.
The capacity of the immune system from TCT patients to respond to IL-2 has important implications for the therapy of HIV-infected patients. Indeed, we show that for TCT patients, CD4 T lymphocytes as well as cells involved in specific and nonspecific cytotoxic responses have the capacity to respond to IL-2, whereas B lymphocytes are less reactive. On the other hand, it seems that TCT does not trigger an increase in IL-2 secretion (M. Sinet, personal communication). These results suggest that IL-2 therapy associated with TCT should facilitate the recovery of the CD4 T lymphocytes of HIV-infected patients and also contribute to the stimulation of protective cellular immunity against opportunistic infections, and eventually, against residual virus reservoir.
Acknowledgments
We are grateful to Dr. M. Sinet for her valuable advice. We particularly acknowledge Dr. B. Gachot for his helpful comments. We are indebted to Dr F. Saul for advice and critical review of the manuscript. We thank J.-L. Lagneau, A. Azar, B. Bénard, M. Minet, T. Perca, and P. Trumbic for their expert technical assistance. This work was supported by grants from the Agence Nationale de Recherches sur le SIDA (Paris), the National Institutes of Health (U.S.A.; Grant CA41619), and Chiron-Europe (Amsterdam). D.D. and L.B. are supported by SIDACTION (Fondation pour la Recherche Médicale, Paris) and by the Agence Nationale de Recherches sur le SIDA, respectively.
ABBREVIATIONS
- IL-2
interleukin-2
- IL-2R
IL-2 receptor, TCT, triple combination therapy
- HAART
highly active antiretroviral therapy
- RT
reverse transcriptase
- NK
natural killer
- PBMC
peripheral blood mononuclear cell(s)
- AZT
3′-azido-3′-deoxythymidine
- DDI
2′,3′-dideoxyinosine
- D4T
2′,3′-didehydro-3′-deoxythymidine
- DDC
2′,3′-dideoxycytidine
- 3TC
2′,3′-dideoxy-3′-thiacytidine
- FITC
fluorescein isothiocyanate
- PE
phycoerythrin
- TC
Tri color
- PHA
phytohemagglutinin
- MFI
mean fluorescence intensity
References
- 1.Smith K A. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 2.Bani L, David D, Moreau J-L, Cayota A, Nakarai T, Ritz J, Thèze J. Int Immunol. 1997;9:573–580. doi: 10.1093/intimm/9.4.573. [DOI] [PubMed] [Google Scholar]
- 3.Caligiuri M A, Zmuidzinas A, Manley T J, Levine H, Smith K A, Ritz J. J Exp Med. 1990;171:1509–1526. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson T D. In: Structure and Function of Interleukin-2. Kunkel S L, Remick D G, editors. New York: Dekker; 1992. pp. 27–60. [Google Scholar]
- 5.Moreau J-L, Chastagner P, Tanaka T, Miyasaka M, Kondo M, Sugamura K, Thèze J. J Immunol. 1995;155:3401–3408. [PubMed] [Google Scholar]
- 6.Thèze J, Alzari P M, Bertoglio J. Immunol Today. 1996;10:481–486. doi: 10.1016/0167-5699(96)10057-c. [DOI] [PubMed] [Google Scholar]
- 7.Bosco M C, Espinoza-Delgado I, Rowe T K, Malabarba M G, Longo D L, Varesio L. J Immunol. 1997;159:2922–2931. [PubMed] [Google Scholar]
- 8.Leonard W J, Shores E W, Love P E. Immunol Rev. 1995;148:97–114. doi: 10.1111/j.1600-065x.1995.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 9.Lai S Y, Xu W, Gaffen S L, Liu K D, Longmore G D, Greene W C, Goldsmith M A. Proc Natl Acad Sci USA. 1996;93:231–235. doi: 10.1073/pnas.93.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, Nakamura M, Takeshita T. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 11.Tagaya Y, Bamford R N, DeFilippis A P, Waldmann T A. Immunity. 1996;4:329–336. doi: 10.1016/s1074-7613(00)80246-0. [DOI] [PubMed] [Google Scholar]
- 12.Poli G, Fauci A S. In: Role of Cytokines in the Pathogenesis of HIV Infection. Aggarwal B B, Puri R K, editors. Cambridge, MA: Blackwell; 1995. pp. 421–449. [Google Scholar]
- 13.Sahraoui Y, Ammar A, Lunardi-Iskandar Y, Tsapis A, Spanakis E, N′Go N, Allouche M, Gay Bellile V, Jasmin C, Georgoulias V. Cell Immunol. 1992;139:318–332. doi: 10.1016/0008-8749(92)90074-y. [DOI] [PubMed] [Google Scholar]
- 14.Chopra R K, Raj N B K, Scally J P, Donnenberg A D, Adler W H, Saah A J. Clin Exp Immunol. 1993;91:18–24. doi: 10.1111/j.1365-2249.1993.tb03347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanham G, Kestens L, Vingerhoets J, Penne G, Colebunders R, Vandenbruaene M L, Goeman J, Ceuppens J L, Sugamura K, Gigase P. Clin Immunol Immunopathol. 1994;71:60–68. doi: 10.1006/clin.1994.1052. [DOI] [PubMed] [Google Scholar]
- 16.David D, Bani L, Moreau J-L, Demaison C, Sun K, Salvucci O, Nakarai T, de Montalembert M, Chouaïb S, Joussemet M, Ritz J, et al. Blood. 1998;91:165–172. [PubMed] [Google Scholar]
- 17.Collier A C, Coombs R W, Schoenfeld D A, Bassett R L, Timpone J, Baruch A, Jones M, Facey K, Whitacre C, McAuliffe V J, Friedman H M, et al. N Engl J Med. 1996;334:1011–1017. doi: 10.1056/NEJM199604183341602. [DOI] [PubMed] [Google Scholar]
- 18.Autran B, Garcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debré P, Leibowitch J. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 19.Kovacs J A, Vogel S, Albert J M, Falloon J, Davey R T, Walker R E, Polis M A, Spooner K, Metcalf J A, Baseler M, et al. N Engl J Med. 1996;335:1350–1356. doi: 10.1056/NEJM199610313351803. [DOI] [PubMed] [Google Scholar]
- 20.Leef Jacobson E, Pilaro F, Smith K A. Proc Natl Acad Sci USA. 1996;93:10405–10410. doi: 10.1073/pnas.93.19.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakarai T, Robertson M J, Streuli M, Wu Z, Ciardelli T L, Smith K A, Ritz J. J Exp Med. 1994;180:241–251. doi: 10.1084/jem.180.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyjek E M, Bartkowiak J, Drozdz R, Wasik T J, Jasinski M, Kaneko Y, Lischner H W, Kozbor D. J Immunol. 1997;158:464–474. [PubMed] [Google Scholar]
- 23.Pericle F, Pinto L A, Hicks S, Kirken R A, Sconocchia G, Rusnak J, Dolan M J, Shearer G M, Segal D M. J Immunol. 1998;160:28–31. [PubMed] [Google Scholar]
- 24.Meyaard L, Hovenkamp E, Keet I P M, Hooibrink B, de Jong I H, Otto S A, Miedema F. J Immunol. 1996;157:2712–2718. [PubMed] [Google Scholar]
- 25.McMichael A J, Phillips R E. Annu Rev Immunol. 1997;15:271–296. doi: 10.1146/annurev.immunol.15.1.271. [DOI] [PubMed] [Google Scholar]
- 26.Hauser G J, Bino T, Rosenberg H, Zakuth V, Geller E, Spirer Z. Clin Exp Immunol. 1984;56:14–17. [PMC free article] [PubMed] [Google Scholar]
- 27.Gruters R A, Terpstra F G, De Jong R, Van Noesel C J M, Van Lier R A W, Miedema F. Eur J Immunol. 1990;20:1039–1044. doi: 10.1002/eji.1830200514. [DOI] [PubMed] [Google Scholar]
- 28.Cayota A, Vuillier F, Siciliano J, Dighiero G. Int Immunol. 1994;6:611–621. doi: 10.1093/intimm/6.4.611. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz R H. Curr Opin Immunol. 1997;9:351–357. doi: 10.1016/s0952-7915(97)80081-7. [DOI] [PubMed] [Google Scholar]
- 30.Bani L, David D, Février M, Pialoux G, Dupont B, Sugamura K, Thèze J. Eur J Immunol. 1997;27:2188–2194. doi: 10.1002/eji.1830270911. [DOI] [PubMed] [Google Scholar]
- 31.Haynes B F, Pantaleo G, Fauci A S. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 32.Gougeon M L, Lecoeur H, Boudet F, Ledru E, Marzabal S, Boullier S, Roue R, Nagata S, Heeney J. J Immunol. 1997;158:2964–2976. [PubMed] [Google Scholar]
- 33.Oyaizu N, Adachi Y, Hashimoto F, McCloskey T W, Hosaka N, Kayagaki N, Yagita H, Pahwa S. J Immunol. 1997;158:2456–2463. [PubMed] [Google Scholar]
- 34.Wong J K, Hezareh M, Günthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 35.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, et al. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 36.Chun T W, Stuyver L, Mizell S B, Ehler L A, Mican J A M, Baseler M, Lloyd A L, Nowak M A, Fauci A S. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]