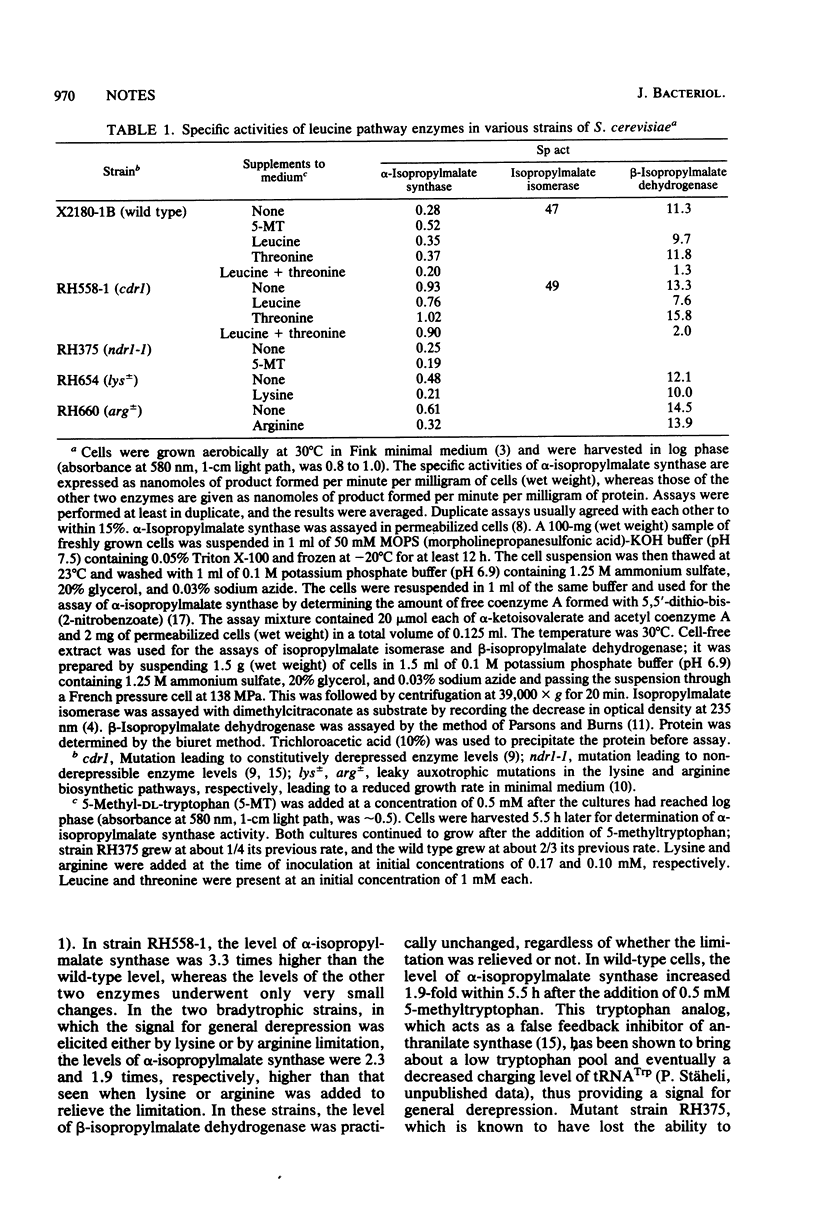
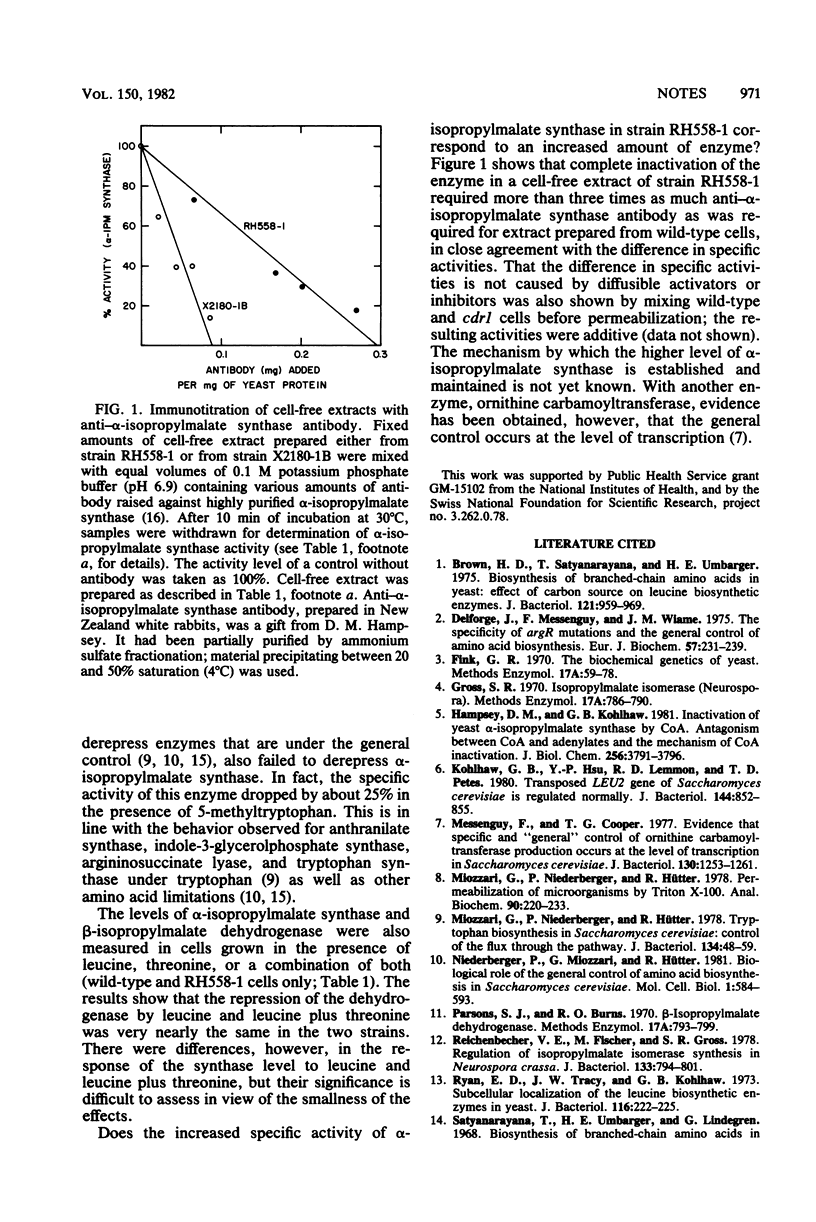
Abstract
The specific activity and the immunoreactive amount of alpha-isopropylmalate synthase were more than three times above wild-type values in a Saccharomyces cerevisiae mutant (cdr1) with constitutively derepressed levels of enzymes known to be under the "general" control of amino acid biosynthesis. The specific activity was also higher in lysine- and arginine-leaky strains when these were grown under limiting conditions, and in wild-type cells grown in the presence of 5-methyltryptophan. A low specific activity was found in a mutant (ndr1) unable to derepress enzymes of the general control system. Neither isopropylmalate isomerase nor beta-isopropylmalate dehydrogenase responded to general control signals.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown H. D., Satyanarayana T., Umbarger H. E. Biosynthesis of branched-chain amino acids in yeast: effect of carbon source on leucine biosynthetic enzymes. J Bacteriol. 1975 Mar;121(3):959–969. doi: 10.1128/jb.121.3.959-969.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delforge J., Messenguy F., Wiame J. M. The regulation of arginine biosynthesis in Saccharomyces cerevisiae. The specificity of argR- mutations and the general control of amino-acid biosynthesis. Eur J Biochem. 1975 Sep 1;57(1):231–239. doi: 10.1111/j.1432-1033.1975.tb02295.x. [DOI] [PubMed] [Google Scholar]
- Hampsey D. M., Kohlhaw G. B. Inactivation of yeast alpha-isopropylmalate synthase by CoA. Antagonism between CoA and adenylates and the mechanism of CoA inactivation. J Biol Chem. 1981 Apr 25;256(8):3791–3796. [PubMed] [Google Scholar]
- Kohlhaw G. B., Hsu Y. P., Lemmon R. D., Petes T. D. Transposed LEU2 gene of Saccharomyces cerevisiae is regulated normally. J Bacteriol. 1980 Nov;144(2):852–855. doi: 10.1128/jb.144.2.852-855.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenguy F., Cooper T. G. Evidence that specific and "general" control of ornithine carbamoyltransferase production occurs at the level of transcription in Saccharomyces cerevisiae. J Bacteriol. 1977 Jun;130(3):1253–1261. doi: 10.1128/jb.130.3.1253-1261.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miozzari G. F., Niederberger P., Hütter R. Permeabilization of microorganisms by Triton X-100. Anal Biochem. 1978 Oct 1;90(1):220–233. doi: 10.1016/0003-2697(78)90026-x. [DOI] [PubMed] [Google Scholar]
- Miozzari G., Niederberger P., Hütter R. Tryptophan biosynthesis in Saccharomyces cerevisiae: control of the flux through the pathway. J Bacteriol. 1978 Apr;134(1):48–59. doi: 10.1128/jb.134.1.48-59.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederberger P., Miozzari G., Hütter R. Biological role of the general control of amino acid biosynthesis in Saccharomyces cerevisiae. Mol Cell Biol. 1981 Jul;1(7):584–593. doi: 10.1128/mcb.1.7.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbecher V. E., Jr, Fischer M., Gross S. R. Regulation of isopropylmalate isomerase synthesis in Neurospora crassa. J Bacteriol. 1978 Feb;133(2):794–801. doi: 10.1128/jb.133.2.794-801.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan E. D., Tracy J. W., Kohlhaw G. B. Subcellular localization of the leucine biosynthetic enzymes in yeast. J Bacteriol. 1973 Oct;116(1):222–225. doi: 10.1128/jb.116.1.222-225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana T., Umbarger H. E., Lindegren G. Biosynthesis of branched-chain amino acids in yeast: regulation of leucine biosynthesis in prototrophic and leucine auxotrophic strains. J Bacteriol. 1968 Dec;96(6):2018–2024. doi: 10.1128/jb.96.6.2018-2024.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy J. W., Kohlhaw G. B. Evidence for two distinct CoA binding sites on yeast alpha-isopropylmalate synthase. J Biol Chem. 1977 Jun 25;252(12):4085–4091. [PubMed] [Google Scholar]
- Ulm E. H., Böhme R., Kohlhaw G. Alpha-isopropylmalate synthase from yeast: purification, kinetic studies, and effect of ligands on stability. J Bacteriol. 1972 Jun;110(3):1118–1126. doi: 10.1128/jb.110.3.1118-1126.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfner M., Yep D., Messenguy F., Fink G. R. Integration of amino acid biosynthesis into the cell cycle of Saccharomyces cerevisiae. J Mol Biol. 1975 Aug 5;96(2):273–290. doi: 10.1016/0022-2836(75)90348-4. [DOI] [PubMed] [Google Scholar]



