Abstract
Interleukin 3 (IL-3) stimulates the proliferation and differentiation of various haematopoietic progenitor cells. Recently, IL-3 and other cytokines were reported to exert a neurotrophic activity and to be associated with neurological disorders, suggesting their complex role in the central nervous system. We now show that overexpression of IL-3 in transgenic mice causes a motor neuron disease with several features of amyotrophic lateral sclerosis and progressive muscular atrophy. These animals exhibit hind limb paralysis at 7 months of age, associated with dendritic and axonal degeneration, loss of motor neurons in the spinal cord, and autoimmune reaction against these cells. We examined the effect of IL-3 on embryonic motor neurons survival in mixed spinal cord cultures. Our results suggest that motor neuronal degeneration is not directly triggered by the high level of expression of IL-3.
Interleukin 3 (IL-3) stimulates the proliferation and/or differentiation of pluripotent haematopoietic stem cells, of various haematopoietic cell lineage progenitors, and of eosinophil and mast cells (1). IL-3 enhances haematopoiesis in numerous animal models (2) and is used in clinical trials to accelerate neutrophil and platelet recovery in patients subjected to myelotoxic chemotherapy or bone marrow transplantation (3).
More recently, IL-3 was shown to be expressed by neurons and astrocytes in mouse brain (4), as well as in primary culture of rodent microglial cells, hippocampal, and septal neurons (5–7). Appel et al. (8) showed that the expression of the βc (signal transducing) subunit shared by IL-3, IL-5, and granulocyte/macrophage colony-stimulating factor receptors can be induced in rat microglial cells in vivo. Moreover, Konishi et al. (9) found an IL-3 receptor-associated antigen immunoreactivity in large neurons of the mouse magnocellular basal nuclei, pyramidal cells of the cerebral cortex, and neuronal cells in some nuclei of the brainstem. These data suggest an active role of IL-3 in the central nervous system (CNS).
Indeed, IL-3 was shown to have a neurotrophic activity for central cholinergic neurons in rodents (4). Some interleukins, including IL-3, have also been associated with both neurodegeneration and repair in the CNS. The synthesis of IL-1, IL-2, IL-3, and IL-6 increases during various neurological disorders, such as multiple sclerosis (MS), Alzheimer’s disease, Parkinson’s disease, as well as during CNS injury and viral infection (4, 10–13). IL-1β and other cytokines, including IL-3, are neurotrophic at low concentration, or neurotoxic at high concentration (11, 14).
To understand the pleiotropic role of IL-3 and address how it exerts its role on the pathogenesis of CNS injury, we and others generated transgenic mice and analyzed their phenotype. Cockayne et al. (15) reported that transgenic mice expressing an antisense RNA for IL-3 exhibited either a lymphoproliferative syndrome or a progressive neurologic dysfunction characterized by abnormal rotatory movement and ataxia. However, the authors were not able to attribute the neurologic dysfunction to gross pathology in the CNS (15). As our studies were in progress, Chiang et al. (16) generated transgenic mice for IL-3 under the control of the glial fibrillary acidic protein gene (GFAP) promoter to target expression of IL-3 to the astrocytes. These mice developed a progressive motor dysfunction characterized, at onset, by impaired rota-rod performance (16). The authors attributed the dysfunction to demyelination induced by activation and recruitment of microglial cells in the brain (16). We developed transgenic mice, referred to as IL3-Tg, which overexpress the mouse IL-3 under the control of a cytomegalovirus (CMV) promoter to achieve a widespread expression of the transgene in various organs. In this paper, we show that the general overexpression of IL-3 in mice leads to a motor neuron (MN) disease with several features of human amyotrophic lateral sclerosis (ALS), and progressive muscular atrophy (PMA). Furthermore, these symptoms are associated with a severe autoimmune reaction against spinal cord MN.
To assess whether IL-3 plays a direct or indirect role on MN degeneration, we examined its effect on the survival and differentiation of MN in a mixed spinal cord culture. Our results suggest that MN degeneration does not result from a direct effect of IL-3 on these cells.
EXPERIMENTAL PROCEDURES
Transgene Construction and Production of Transgenic Mice.
The 1.3-kb EcoRI fragment containing the entire murine IL-3 cDNA and its polyadenylation signal was inserted into EcoRI-linearized pcDNAI vector under the regulatory elements of a CMV promoter to generate the recombinant fragment CMV–IL-3. The fusion gene was introduced into fertile one-cell FVB/n mouse embryos. F1 founder animals were screened for the IL-3 transgene by using PCR. The two PCR oligonucleotides used for the analysis of the transgene were 5′-AGCTCTACCACCAGCATCCACACCATGCTG-3′ on the transcribed strand and 5′-CGGTTAGGAGAGACGGAGCCAGATGCGGGC-3′ on the opposite strand. To confirm the PCR results, we performed a Southern blot analysis with genomic mouse DNA prepared from tail samples. DNA samples were digested with XbaI, separated on agarose gel, and transferred onto nylon membrane by using standard procedures. A high-specific activity 32P-labeled DNA probe encompassing the full-length of IL-3 coding region was synthesized by the random priming method by using [32P]dCTP.
Analysis of IL-3 Expression.
To examine the expression of the transgene we performed a reverse transcriptase-PCR (RT-PCR) and an ELISA.
RT-PCR.
To assess the expression of the transgene in both IL3-Tg and control littermates, we carried out a RT-PCR. Total cellular RNA was isolated from various tissues by the guanidinium isothiocyanate method (17). The RNA was reverse transcribed by using the StrataScript RT-PCR kit (Stratagene) according to the manufacturer’s directions. Five microliters of the first strand were amplified by PCR, using the same set of primers mentioned above and used to identify the transgenic animals. Specific primers to the actin housekeeping gene were also used in the RT-PCR to monitor the quality and quantity of RNA in each samples. Equal volumes from amplified material were loaded onto an agarose gel. The amplified PCR products were then transferred onto a nylon membrane and probed with a radiolabeled 32P-specific IL-3 probe as indicated above. Detection of the transgene was performed by autoradiography using X-Omat Kodak films.
ELISA.
Tissue samples from transgenic and control mice were homogenized at 4°C in 50 mM Tris⋅HCl (pH 7.5), 1% Nonidet P-40, 2 mM EDTA, 100 mM NaCl containing protease inhibitors (20 μg/ml aprotinin and leupeptin/1 mM phenylmethylsulfonyl fluoride). The samples were then centrifuged at 14,000 × g, and the supernatant was used for protein quantification and determination of IL-3 protein expression by double-sandwich ELISA. Two specific mAbs for murine IL-3 (clones MP2-8F8 and MP2-43D11) were used according to manufacturer instructions (PharMingen).
Histological Examination and Immunohistochemistry.
The brain and spinal cord from transgenic and nontransgenic mice were fixed overnight in 10% phosphate-buffered formalin, processed by paraffin infiltration in longitudinal (brain) or coronal orientation (spinal cord), except as indicated in figure legends. Sections were cut on a rotary microtome at 6–8 μm and examined after staining with hematoxylin/eosin or Nissl dyes. For immunohistochemical studies, the tissue sections were deparaffinized and rehydrated through xylene and graded alcohol. Sections were then permeabilized for 60 min at room temperature with PBS plus 0.2% Triton X-100 and 10% normal horse serum. Immunohistochemical studies were performed by using mouse mAbs against microtubule associated protein 2 (MAP-2; Sigma; 1:500), 200-kDa neurofilament proteins (NF200; Sigma; 1:200), GFAP (ICN; 1:200), and choline acetyltransferase (ChAT; Chemicon International, Temecula, CA; 1:100).
The number of MN was evaluated by counting large ChAT-positive cells, having a distinct nucleus, in the lateroventral horns of L4–L5 segments of the lumbar spinal cord. A Nissl-staining analysis was also used to confirm the counting of MN. Neuronal cells were counted in alternate serial sections to avoid that the same cell would be counted twice. We counted a total of 78 sections from four controls, and a total of 46 sections from four transgenic mice. Morphometric measurements of cross-sectional area of 85 MN per group was performed by using the neurolucinda software (Microbrightfield, Colchester, VT).
Autoantibodies Titer and Specificity.
To detect autoimmune reactions, lumbar spinal cord sections of control mice were incubated as indicated above with a 1:20 dilution of the serum from IL3-Tg or age-matched controls. The detection of primary antibodies was performed by using avidin-biotin-peroxidase techniques and diaminobenzidine (Vector Laboratories).
Spinal Cord Cultures.
Mixed spinal cord cultures were prepared from 13-day-old mouse embryos, as described (18), with the following modification. The cells were plated on polyornithine-fibronectin-coated 24-well plates (2 × 105 cells/cm2), in DMEM with 10−6 M human apotransferin, 10−4 M putrescine, 2 × 10−8 M progesterone, 3 × 10−8 M sodium selenite, 4 × 10−6 M bovine insulin, and 10% heat inactivated fetal bovine serum. After exposure to 10−5 M of cytosine arabinoside to arrest nonneuronal cell division, the cells were transferred for 6–9 days to serum-free medium supplemented with 1 μg/ml of recombinant mouse IL-3 (Genzyme), with addition of fresh IL-3 solution every 3 days.
Cells were immunostained with the SMI-32 antibody (Sternberger Monoclonals, Baltimore), a specific marker for nonphosphorylated neurofilaments of MN in spinal cord slices and dissociated spinal cultures (18).
RESULTS
Generation of Transgenic Mice and Expression of IL-3 Transgene.
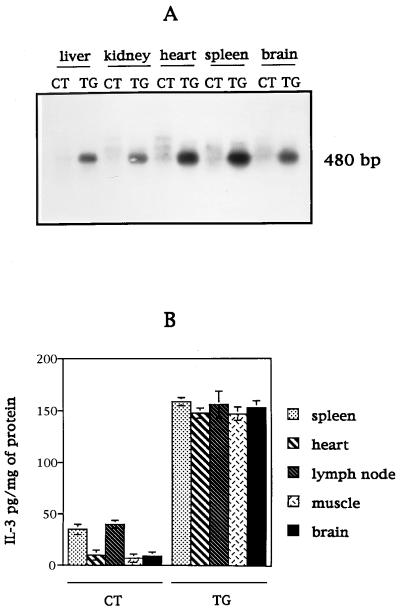
We have generated transgenic mice expressing the IL-3 protein under the control of a CMV promoter. This promoter was used to achieve a widespread expression of the transgene in various organs, in contrast to a highly targeted expression using a tissue-specific promoter, such as GFAP. The PCR analysis of tail DNA from various F1 animals showed the presence of a band of the expected size (480 bp). Stable integration of the transgene was also demonstrated in all transgenic mice analyzed by Southern blot analysis of genomic DNA (data not shown). To monitor the expression of the IL-3 transgene, we performed a RT-PCR analysis and a double-sandwich ELISA assay with protein extracts from various tissues of transgenic and age-matched controls. The RT-PCR analysis of mRNA showed the expected amplified product of 480 bp in transgenic animals only, whereas the level of expression of the actin housekeeping gene was similar between IL3-Tg and controls (data not shown). To verify the specificity of the amplified product, the agarose gel was transferred onto a nylon membrane and hybridized with a specific radiolabeled probe for IL-3 (Fig. 1A). The RT-PCR product corresponding to the IL-3 transgene was detected in all examined organs of IL3-Tg mice (Fig. 1A, TG lanes); this product was not detected in control tissues (Fig. 1A, CT lanes).
Figure 1.
Determination of IL-3 transgene integration and expression in F1 intermating mice. (A) mRNA from various tissues of IL3-Tg (TG) and controls (CT) were analyzed by RT-PCR for IL-3 expression by using specific primers. The RT-PCR product was loaded onto an agarose gel, transferred onto nylon membrane and probed with a radiolabeled-specific IL-3 probe. (B) The level of IL-3 protein expression in various organs of IL3-Tg (TG) or controls (CT) was determined by ELISA. Data from four mice per group are expressed as mean ± SD.
The expression of the IL-3 protein was quantitated in four transgenic and four age-matched controls (Fig. 1B). The results showed a 4-fold increase of IL-3 level in transgenic compared with control organs where the cytokine is normally expressed (spleen and lymph nodes), and a 15-fold elevation in transgenic compared with control organs where IL-3 is not highly expressed (heart, muscle, brain).
The observation of a severe neuronal pathology in the spinal cord of IL3-Tg mice prompted us to examine the expression of the IL-3 transgene and protein in this tissue. Our data showed that the level of IL-3 expression in the spinal cord, both at the RNA and protein levels, was quite similar to that observed in the brain of IL3-Tg (data not shown).
IL3-Tg Develop Neuronal Degeneration in the Brainstem and Spinal Cord.
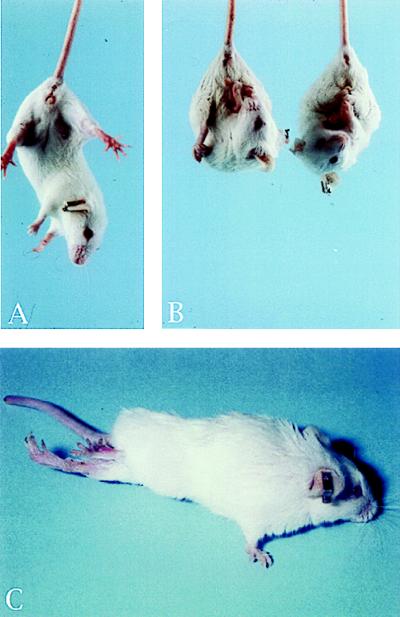
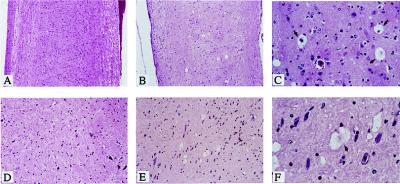
Transgenic mice and their offsprings derived from the two founder lines were housed in a pathogen-free animal facility in the same conditions as other transgenic lines. They appeared normal during the first few months of postnatal development, and no histological abnormalities were detected. Beginning at 7 months of age, the mice manifested signs of neurological abnormality. All affected mice exhibited tremor, rough coat, and weight loss when compared with age-matched controls. When lifted by the tails, IL-3 transgenic mice reflexively contracted their limbs (Fig. 2B), whereas normal mice extended their legs (Fig. 2A). The disease was progressive, culminating with severe paralysis of the hind limbs and less commonly, the forelimbs (Fig. 2C). Affected mice were no longer able to forage for food or water and were sacrificed for histologic examination. The end-stage of the disease, leading to the sacrifice of animals occurred at ≈10 months for the majority of them. This clinical phenotype occurred in transgenic mice derived from two independent transgenic lines. This fact indicates that the pathology resulted specifically from IL-3 transgene expression. Histological examination of spinal cord and brain sections from IL3-Tg revealed multifocal neuronal degeneration, characterized by vacuolation of neuronal cytoplasm mainly in the brainstem and throughout the spinal cord (Fig. 3 B and C, E and F), whereas no obvious histologic abnormalities were observed in spinal cord and brainstem of age-matched controls (Fig. 3 A and D).
Figure 2.
IL3-Tg develop paralysis of hind limbs. (A) Normal mice. (B and C) IL3-Tg. When lifted by their tails, IL3-Tg reflexively contract their limbs whereas controls extend their legs (A and B). At later stage, they exhibit severe hind limbs paralysis (C).
Figure 3.
Presence of vacuolated neurons in the brainstem and spinal cord of IL3-Tg. Longitudinal sections of formalin-fixed, paraffin embedded spinal cord (A, B, and C) and brainstem (D, E, and F) from an 8-month-old IL3-Tg (B and C, E and F) or age-matched control mice (A and D) were stained with hematoxylin/eosin. C and F are higher magnification views (×63) of B and E (×10), respectively, whereas A and D are ×10. Arrows indicate vacuolated neurons.
The paralysis of hind limbs exhibited by IL3-Tg mice prompted us to analyze the phenotype of MN in the spinal cord. We monitored the immunoreactivity of lumbar spinal cord sections for cholinergic neuron antigens by using a specific antibody for ChAT. The cell count of Nissl and ChAT-immunostained sections revealed a 35% (P < 0.05) and 45% (P < 0.001) loss, respectively, of lumbar spinal cord MN in IL3-Tg mice compared with controls (Table 1). The remaining MN in transgenic mice were generally shrunken, hyperchromatic by light microscopy, suggesting a degenerative process (Fig. 4 A–D). The slightly higher background for the transgenic sections (Fig. 4 B and D) is caused by the longer incubation time used to increase the labeling of the remaining MN. We performed a morphometric analysis of lumbar spinal cord MN. Cross-sectional area of MN was 47% lower in IL3-Tg (182.4 ± 6.8 μm2; P < 0.001) compared with age-matched controls (343.6 ± 13.4 μm2). MN degeneration and loss were most severe in the lumbar (L4, L5) and sacral region, less severe in the thoracic region, and very low in the cervical region of the spinal cord (data not shown). In addition, we did not observe any significant loss of MN in the facial, hypoglossal, and trigeminal motor nuclei of the brainstem (data not shown).
Table 1.
Loss of MN in the spinal cord of IL3-Tg mice
| Mice | Number of sections | Staining | MN count (mean ± SD) |
|---|---|---|---|
| CT | 50 | Nissl | 47.9 ± 8 |
| IL3-Tg | 27 | Nissl | 31.3 ± 6.1 |
| CT | 28 | ChAT | 36.2 ± 4.5 |
| IL3-Tg | 19 | ChAT | 19.5 ± 6.2 |
Coronal sections from the lumbar spinal cord (L4–L5) of four 10-month-old transgenic (IL3-Tg) and four age-matched controls (CT) were either immunolabeled with ChAT antibodies or stained with the Nissl dye. The total number of sections used for the determination of MN number ± SD is indicated. Both Nissl and ChAT staining showed significant differences in MN counts between IL3-Tg and controls, using the Student’s t test (P < 0.05 for Nissl values; P < 0.001 for ChAT values).
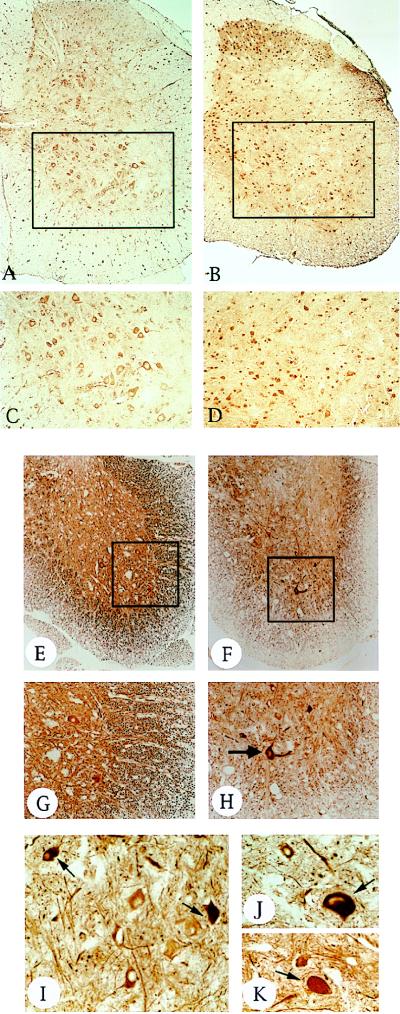
Figure 4.
IL3-Tg exhibit a loss of MN and axonal degeneration in the spinal cord. (A–D) ChAT immunoreactivity (ChAT antibody, 1:100) of coronal sections from a 10-month-old control (A and C) or transgenic lumbar spinal cord (B and D). C and D are higher magnifications (×20) of selected areas in A and B (×10), respectively. In addition to their shrunken size, there is a reduced number of ChAT-positive MN in IL3-Tg (B and D) compared with controls (A and C). (E–K) NF200 immunoreactivity (NF200 antibody 1:200) of coronal sections from an 11-month-old control (E and G) or transgenic (F and H–K) lumbar spinal cord. G and H are higher magnification views (×20) of E and F (×10), respectively. I–K are ×40 magnifications of various sections of the same transgenic animal. Note the axonal neuronal degeneration in transgenic (H) compared with controls (G), as monitored by the dramatic loss of NF200 staining. The arrows in H–K show the accumulation of neurofilaments in the neuronal cell body and proximal axons of some MN in IL3-Tg. Similar accumulations were also observed at earlier stages of the disease (i.e., presenting a milder paralysis of limbs).
To evaluate the extent of damage to neuronal processes, we examined the immunoreactivity of lumbar spinal cord sections for MAP-2 and NF200, which are specifically localized in dendrites and axons, respectively. A substantial reduction of neurofilament immunoreactivity, suggesting axonal degeneration occurred in IL3-Tg mice (Fig. 4F) compared with controls (Fig. 4E). The axonal complexity was also markedly decreased in IL3-Tg mice (Fig. 4H). Similar results were found for dendrites by using MAP-2 antibodies (data not shown). An increase in NF200 immunoreactivity in perikaria and proximal axons of remaining MN suggests an accumulation of neurofilaments in these sites (Fig. 4 H–K).
The targeted expression of IL-3 in astrocytes (Chiang’s model) induces a severe demyelination process in the brain of transgenic animals (16). To document the presence of a similar phenotype in our animal model, we conducted a histological examination of myelin in spinal cord sections of IL3-Tg and controls by using the luxol fast blue staining. Our data showed no significant differences between both groups in the dense blue staining of myelin in the white matter (data not shown).
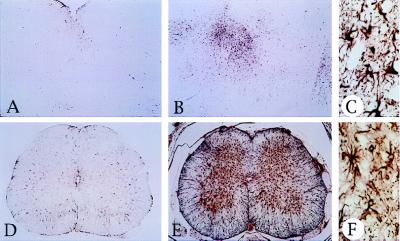
Inflammation, injury and neurodegeneration of the CNS induce reactive astrocytosis, characterized by an increase of GFAP-positive cells. We found that GFAP immunoreactivity was markedly increased in the spinal cord and brainstem of IL3-Tg mice (Fig. 5 E and B) compared with controls (Fig. 5 D and A). In addition, GFAP-positive astrocytes were hypertrophied (Fig. 5 C and F).
Figure 5.
Reactive astrocytosis in IL3-Tg. GFAP immunoreactivity (GFAP antibody, 1:200) of control (A and D) and transgenic (B, C, E, and F) brainstem (A–C) or spinal cord (D–F) sections. C and F are higher magnification views (×40) of B and E (×5), respectively. Note the proliferation of GFAP-positive astrocytes in IL3-Tg (B and E), compared with controls (A and D). The brainstem and spinal cord sections were taken from 7-month-old mice.
Neuronal Degeneration of the CA1 Hippocampal Layer in IL3-Tg.
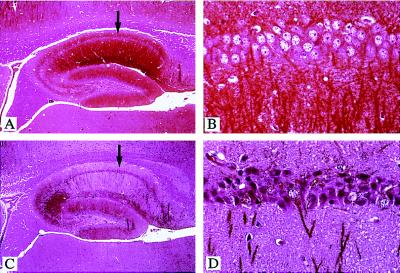
Immunohistochemical analysis of brain sections, using MAP-2-specific antibodies, showed a marked loss of dendrites in the hippocampus of IL3-Tg, as compared with controls (Fig. 6 A and C). Dendritic loss was mainly localized to pyramidal cells of the CA1 layer, whereas in the most severe cases, other regions of the hippocampus, such as CA2 and CA3 cell layers and granule cells of the dentate gyrus, were affected (Fig. 6C). In some animals, a reduction of MAP-2 staining was also observed in the motor cortex, whereas other areas, such as the cerebellum, were never affected (data not shown). In the most severe cases, CA1 pyramidal cell bodies from IL3-Tg mice appeared smaller, darker, and more condensed than those of controls (Fig. 6 B and D), suggestive of cellular degeneration. However, there was neither evidence of extensive cellular loss, nor axonal degeneration or reactive astrocytosis (using NF200 or GFAP staining, respectively) in IL3-Tg hippocampi (data not shown).
Figure 6.
Degeneration of pyramidal cells in the hippocampus of IL3-Tg. Longitudinal sections of an 8-month-old control (A and B) or transgenic brain (C and D) were immunolabeled with MAP-2 antibodies (1:500) and counterstained with hematoxylin/eosin. B and D are higher magnification views (×40) of areas indicated by arrows in A and C (×5), respectively.
No axonal loss was detected in other examined brain areas of IL3-Tg mice, including the cerebellum and the cortex. In addition, there was no significant demyelination process in the brain of IL3-Tg, as evidenced by luxol fast blue staining. Careful examination of pyramidal cells of the motor cortex, cerebellar granule cells or Purkinje cells (calbindin D-positive cells), and dopaminergic neurons of the substantia nigra (tyrosine hydroxylase-positive cells) failed to show any abnormal changes in IL3-Tg compared with controls (data not shown).
Evidence of Autoimmune Reactions in MN Degeneration.
Autoimmune reactions have been suggested to represent some of the last events leading to MN diseases (19–21). Indeed, the serum of eight 14-month-old transgenic and three age-matched controls were analyzed for the titer of the following Ig isotypes, IgG1, IgG2a, and IgE, by double-sandwich ELISA. All tested IL3-Tg had an increased titer, up to 8-fold, in either one, two, or all of the three tested Ig isotypes as compared with controls, hinting to a possible autoimmune reaction. IgG2a and IgG1 titers were increased in 90% of IL3-Tg mice analyzed, whereas the IgE titer was increased in 75% of them.
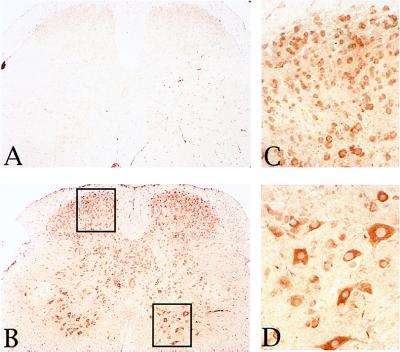
To test the hypothesis of the involvement of autoimmunity in the pathology of IL3-Tg, we performed an immunostaining by using spinal cord tissue sections from wild-type animals and sera from various IL3-Tg or controls. The results in Fig. 7 show a strong immunoreaction for neuronal cells in spinal cord sections incubated with a 1:20 dilution of sera from IL3-Tg (Fig. 7 B–D) as compared with control serum (Fig. 7A). Sensory neurons and particularly MN in the ventral horn were the main target of this autoimmune reaction (Fig. 7 C and D). The same intensity and pattern of immunostaining was obtained with a range of serum dilution from 1:20 to 1/100. In brain sections, similar dilutions of sera from IL3-Tg mice resulted in a weaker and more heterogeneous pattern of reactivity compared with what was observed in spinal cord sections. These results are consistent with the less severe pathology observed in the brain, as compared with the spinal cord of IL3-Tg.
Figure 7.
Autoimmune reactions against spinal cord cells. Lumbar spinal cord sections of control mice were reacted with a 1:20 dilution of the serum from a control (A) or IL3-Tg mouse (B–D). Detection of primary antibodies was done by using the avidin-biotin-peroxidase technique and diaminobenzidine. C and D are higher magnification views (×40) of areas highlighted by the left or right rectangle, respectively (×10). Sensory neurons (C) and MN (D) are the main targets of the autoimmune reaction.
Effect of IL-3 Exposure on Mixed Spinal Cord Neurons.
To determine whether the MN degeneration observed in IL-3-Tg resulted from a direct or indirect effect of the expression of IL-3, we studied the impact of the presence of this lymphokine on mixed embryonic spinal cord cultures. It is widely accepted that neurons prepared from embryonic spinal cord acquire properties of mature neurons in tissue culture. To monitor the neuronal survival, we used the SMI-32 antibody as a specific marker of MN in dissociated spinal cultures (18). Our results showed that the presence of IL-3 for 6–9 days at a concentration up to 1 μg/ml affects neither the survival of MN, nor their differentiation as evidenced by the number of SMI-32 positive cells and neurites in control and treated cultures (data not shown).
DISCUSSION
The systemic overexpression of IL-3 in our transgenic mice results in a severe neurological dysfunction and a MN disease. The clinical features were neither observed in age-matched controls nor in transgenic mice expressing granulocyte/macrophage colony-stimulating factor or IL-5 under the control of the CMV promoter, housed under the same conditions (data not shown). This finding indicates that the pathology of IL3-Tg is a primary consequence of the transgene overexpression.
IL3-Tg exhibited significant dendritic changes associated, in the most severe cases, with pyramidal cell degeneration in the CA1 layer of the hippocampus, similar to that described in transgenic mice overexpressing IL-6 (22) and in animal models of brain ischemia (23). This clinical pathology has been described in Creuzfeldt–Jacob diseases (24) and in HIV encephalitis (25). We observed multifocal neuronal vacuolations in both brainstem and throughout the spinal cord as well as reactive astrocytosis. In addition, IL3-Tg exhibited axonal and dendritic degeneration, and loss of MN in the ventral horn of lumbar (L4, L5) spinal cords, leading to severe hind limb paralysis. These pathological findings are reminiscent of MN diseases such as ALS, and PMA (26, 27). We observed a preferential degeneration of lower MN in the spinal cord of IL3-Tg. However, MN from various motor nuclei in the brainstem and upper MN (i.e., pyramidal cells in the motor cortex and associated corticospinal tracts) were spared. These are common features of PMA, in contrast to ALS, in which both upper and lower MN are generally affected. Nevertheless, the loss of dendrites observed in the motor cortex of some IL3-Tg suggest that involvement of pyramidal cells, in this area, could be a later event in the course of this disease, as shown in some ALS patients.
Unlike ALS and PMA patients whose neurodegeneration is limited to MNs, pyramidal cells in the hippocampus, which control memory and learning, are also affected in IL3-Tg. However, the extent of damages occurring in this area was not as dramatic as that observed in MN degeneration.
The loss of integrity of dendrites and axons in the spinal cord of IL3-Tg, which was mainly observed in the ventral area, suggest that other cells than MN, such as interneurons, could be ultimately affected in this disease. However, it is clear, based on our pathological and immunological studies, that MN are the major affected cells in the spinal cord of our animal model.
The vacuolar degeneration of ventral horn cells and their processes at early stages of the disease, and the loss of lower MN and axons in the later stages culminating in paralysis, were also described in ALS-linked animal models expressing mutant forms of Cu/Zn-superoxide dismutase gene (SOD) (28–30). In addition, transgenic mice overexpressing the light or heavy chains of neurofilament had mild motor dysfunction, associated or not with a significant loss of lower MN (28).
Accumulation of neurofilament proteins in MN cell bodies and proximal axons exhibiting neurodegeneration, which is a hallmark of human ALS, was observed both in IL3-Tg and in ALS-linked transgenic models (28, 30).
Chiang et al. (16) described transgenic mice overexpressing IL-3 under the control of the specific brain promoter GFAP (IL3-GFAP). These transgenic animals developed a progressive motor disorder, beginning at 5 months of age, characterized by impaired rota-rod performance. By light microscopy, these mice had multifocal, plaque-like white matter lesions in the cerebellum and brainstem consequent to demyelination. These animals showed a recruitment of macrophage/microglial cells which was presumed to be the cause of the demyelination process occurring in IL3-GFAP mice.
Chiang’s and our IL3-Tg mice exhibit common features, such as late onset of neuronal pathology and motor disorder. However, they are also distinct differences. (i) Our IL3-Tg manifest a MN disease with ALS-like pathology whereas IL3-GFAP show striking resemblance with inflammatory demyelinating disease such as MS. In this later model, the degeneration was largely limited to the cerebellum, with disorganization of the structure of the granular layer and loss of Purkinje cells. (ii) As evidenced by luxol fast blue staining of spinal cord and brain sections, no demyelination process was evident in IL3-Tg, in contrast to IL3-GFAP. (iii) We found a strong autoimmune reaction against spinal cord neurons including MN in IL3-Tg.
The distinct pathology between Chiang’s model and ours is likely caused by general versus located expression of IL-3 in transgenics. However, both models are affected in their neuronal cells, which demonstrates that the overexpression of IL-3 leads to neurological disturbances.
In humans, about 10% of ALS cases are familial with autosomal dominant inheritance; the majority of cases are sporadic. In some cases, familial ALS has been linked to mutations in the superoxide dismutase gene. However, the cause of most forms of familial and sporadic ALS, and the molecular mechanisms underlying these clinical symptoms, remain unknown.
It has been described that certain lymphokines including IL-3 exert both trophic and toxic effects on neuronal cells (4, 11), and therefore could play a role in a human pathology such as ALS. Because IL3-Tg developed a progressive MN degeneration, we hypothesized that IL-3 might exert a toxic effect on MN leading to their degeneration. To examine this hypothesis, we studied the impact of IL-3 on embryonic spinal cord cultures. Surprisingly, IL-3-treated MN were affected neither in their survival nor in their differentiation, at a concentration up to 1 μg/ml, which was shown to affect the survival of hippocampal cultures (14). Such differences could be explained either by the fact that the toxic effect of IL-3 differs, according to neuronal cell types and their developmental stage, or that MN degeneration is not directly triggered by the expression of IL-3. Nevertheless, our transgenic model demonstrates that overproduction of IL-3, in vivo, could be a contributing factor in the generation of MN disease. This assumption is supported by the fact that the level of cytokines such as IL-1, IL-2, IL-3, or IL-6 is substantially elevated in several neurodegenerative diseases, such as MS and Alzheimer’s disease (4, 10–13).
The final cascade of events leading to MN degeneration is believed to be multifactorial and to involve mechanisms, such as exitotoxicity, free radical accumulation, and possibly immunological disturbances (19–21). Although there have been no data yet showing the involvement of cytokines in ALS-like syndromes, autoimmune reactions have been associated with this disease (19, 21). We have found a substantial increase of serum Igs in IL3-Tg mice compared with controls and have shown the production of autoantibodies against normal spinal cord neuronal antigens. Nevertheless, the cascade of events and the mechanism by which this specific autoimmune reaction is induced in our animal model, as in ALS patients, is still unclear. Indeed, it is not known if autoimmune reactions are the cause or the result of the MN degeneration.
We showed that neurodegeneration in IL3-Tg was characterized mainly by the loss of MN in the spinal cord. The clinical signs exhibited by our transgenic animals were similar to human ALS or PMA. Our results present evidence that the general overproduction of a cytokine, such as IL-3, may play a role, even somewhat indirect, in the generation of an ALS-like pathology. Although more studies will be necessary to further characterize the molecular targets of the autoimmune reactions in IL3-Tg and to dissect the molecular events triggered by IL-3 overproduction in these animals, we believe that our model will be very useful to understand the molecular mechanisms involved during ALS and similar neurodegenerative disorders.
Acknowledgments
We thank Ms. N. Newman for technical assistance and Dr. E. Wawarousek for help with transgenics. Animals were cared for in accordance with institutional guidelines. This work was also supported by the Association Francaise contre les Myopathies (France).
ABBREVIATIONS
- IL
interleukin
- CNS
central nervous system
- MS
multiple sclerosis
- GFAP
glial fibrillary acidic protein
- CMV
cytomegalovirus
- MN
motor neuron
- ALS
amyotrophic lateral sclerosis
- PMA
progressive muscular atrophy
- RT-PCR
reverse transcriptase–PCR
- MAP-2
microtubule associated protein 2
- ChAT
choline-acetyltransferase
- NF200
neurofilament protein
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Schwarzmeier J D. Eur J Haematol. 1996;57:69–74. doi: 10.1111/j.1600-0609.1996.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 2.Metcalf D, Begley C G, Johnson G R, Nicola N A, Lopez A F, Williamson D J. Blood. 1986;68:46–57. [PubMed] [Google Scholar]
- 3.Gianella-Borradori, A. (1994) Stem Cells 12 (Suppl. 1), 241–248. [DOI] [PubMed]
- 4.Sei Y, Vitkovic L, Yokoyama M M. Neuroimmunomodulation. 1995;2:121–133. doi: 10.1159/000096881. [DOI] [PubMed] [Google Scholar]
- 5.Gebicke-Haerter P J, Appel K, Taylor G D, Schobert A, Rich I N, Northoff H, Berger M. J Neuroimmunol. 1994;50:203–14. doi: 10.1016/0165-5728(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 6.Konishi Y, Kamegai M, Takahashi K, Kunishita T, Tabira T. Neurosci Lett. 1994;182:271–274. doi: 10.1016/0304-3940(94)90814-1. [DOI] [PubMed] [Google Scholar]
- 7.Appel K, Honegger P, Gebicke-Haerter P J. J Neuroimmunol. 1995;60:83–91. doi: 10.1016/0165-5728(95)00057-9. [DOI] [PubMed] [Google Scholar]
- 8.Appel K, Buttini M, Sauter A, Gebicke-Haerter P J. J Neurosci. 1995;15:5800–5809. doi: 10.1523/JNEUROSCI.15-08-05800.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konishi Y, Chui D H, Kunishita T, Yamamura T, Higashi Y, Tabira T. J Neurosci Res. 1995;41:572–582. doi: 10.1002/jnr.490410503. [DOI] [PubMed] [Google Scholar]
- 10.Araujo D M, Lapchak P A. Neuroscience. 1994;61:745–754. doi: 10.1016/0306-4522(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 11.Rothwell N J, Strijbos P J L M. Int J Dev Neurosci. 1995;13:179–185. doi: 10.1016/0736-5748(95)00018-c. [DOI] [PubMed] [Google Scholar]
- 12.Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T. Neurosci Lett. 1996;211:13–16. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- 13.Navikas V, Link H. J Neurosci Res. 1996;45:322–333. doi: 10.1002/(SICI)1097-4547(19960815)45:4<322::AID-JNR1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 14.Araujo D M, Cotman C W. Brain Res. 1993;600:49–55. doi: 10.1016/0006-8993(93)90400-h. [DOI] [PubMed] [Google Scholar]
- 15.Cockayne D A, Bodine D M, Cline A, Nienhuis A W, Dunbar C E. Blood. 1994;84:2699–2710. [PubMed] [Google Scholar]
- 16.Chiang C S, Powell H C, Gold L H, Samimi A, Campbell I L. J Clin Invest. 1996;97:1512–1524. doi: 10.1172/JCI118574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maniatis T, Fritsch E F, Sambrook T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1982. [Google Scholar]
- 18.Carriedo S G, Yin H Z, Weiss J H. J Neurosci. 1996;16:4069–4070. doi: 10.1523/JNEUROSCI.16-13-04069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antel J P, Cashman N R. Springer Semin Immunopathol. 1995;17:17–28. doi: 10.1007/BF00194097. [DOI] [PubMed] [Google Scholar]
- 20.Eisen A. Muscle Nerve. 1995;18:741–752. doi: 10.1002/mus.880180711. [DOI] [PubMed] [Google Scholar]
- 21.Smith R G, Appel S H. Annu Rev Med. 1995;46:133–45. doi: 10.1146/annurev.med.46.1.133. [DOI] [PubMed] [Google Scholar]
- 22.Campbell I L, Abraham C R, Masliah E, Kemper P, Inglis J D, Oldstone M B, Mucke L. Proc Natl Acad Sci USA. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araki T, Kato H, Fujiwara T, Kogure K, Itoyama Y. Eur J Pharmacol. 1995;278:91–96. doi: 10.1016/0014-2999(95)00058-s. [DOI] [PubMed] [Google Scholar]
- 24.Ferrer I, Costa F, Veciana J M. Neuropathol Appl Neurobiol. 1981;7:237–242. doi: 10.1111/j.1365-2990.1981.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 25.Masliah E, Achim C L, Ge N, DeTeresa R, Terry R D, Wiley C A. Ann Neurol. 1992;32:321–329. doi: 10.1002/ana.410320304. [DOI] [PubMed] [Google Scholar]
- 26.Rossi M. In: Motor Neuron Disease. Williams A C, editor. London: Chapman & Hall; 1994. pp. 307–341. [Google Scholar]
- 27.Schiffer, D., Cordera, S., Cavalla, P. & Migheli, A. (1996) J. Neurol. Sci. 139 (Suppl.), 27–33. [DOI] [PubMed]
- 28.Higgins L S, Cordell B. Neurodegeneration. 1995;4:117–129. doi: 10.1006/neur.1995.0015. [DOI] [PubMed] [Google Scholar]
- 29.Ripps M E, Huntley G W, Hof P R, Morrison J H, Gordon J W. Proc Natl Acad Sci USA. 1995;92:689–693. doi: 10.1073/pnas.92.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tu P H, Raju P, Robinson K A, Gurney M E, Trojanowski J Q, Lee V M. Proc Natl Acad Sci USA. 1996;93:3155–3160. doi: 10.1073/pnas.93.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]