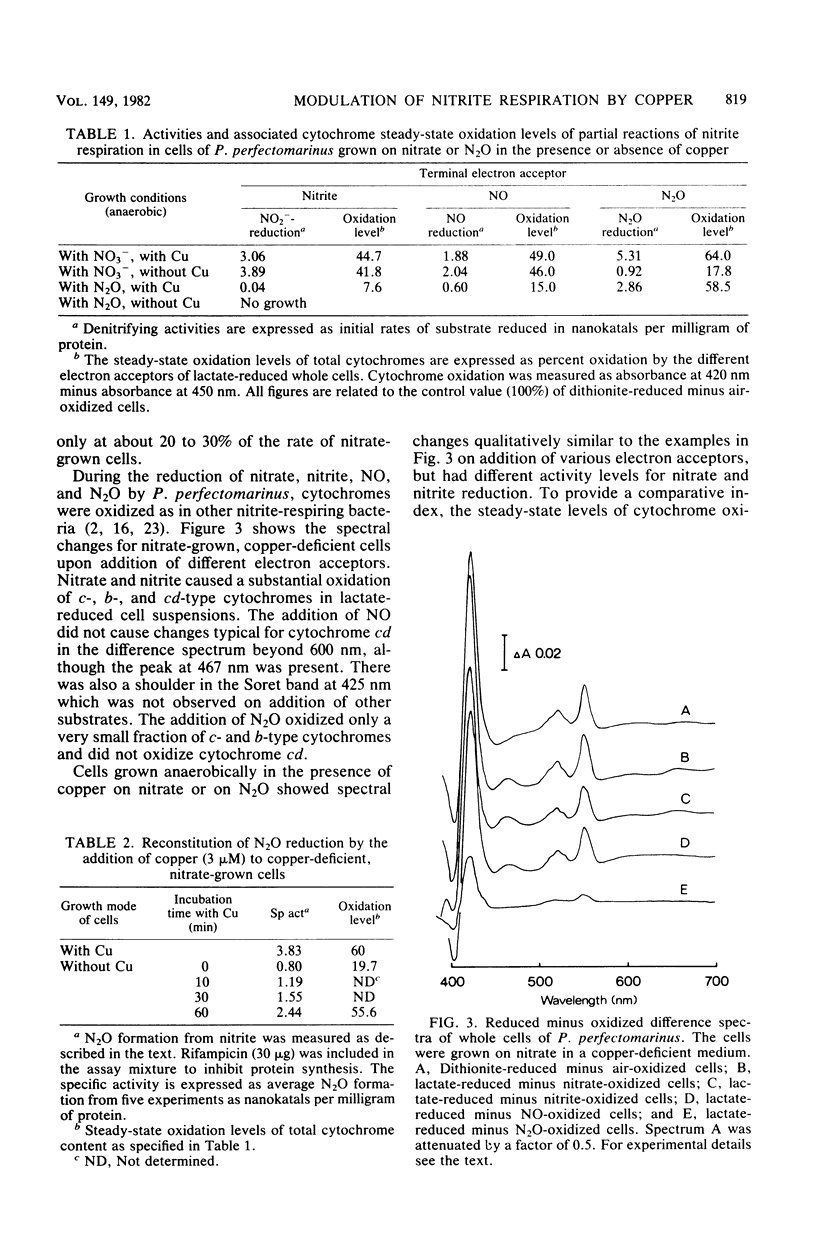
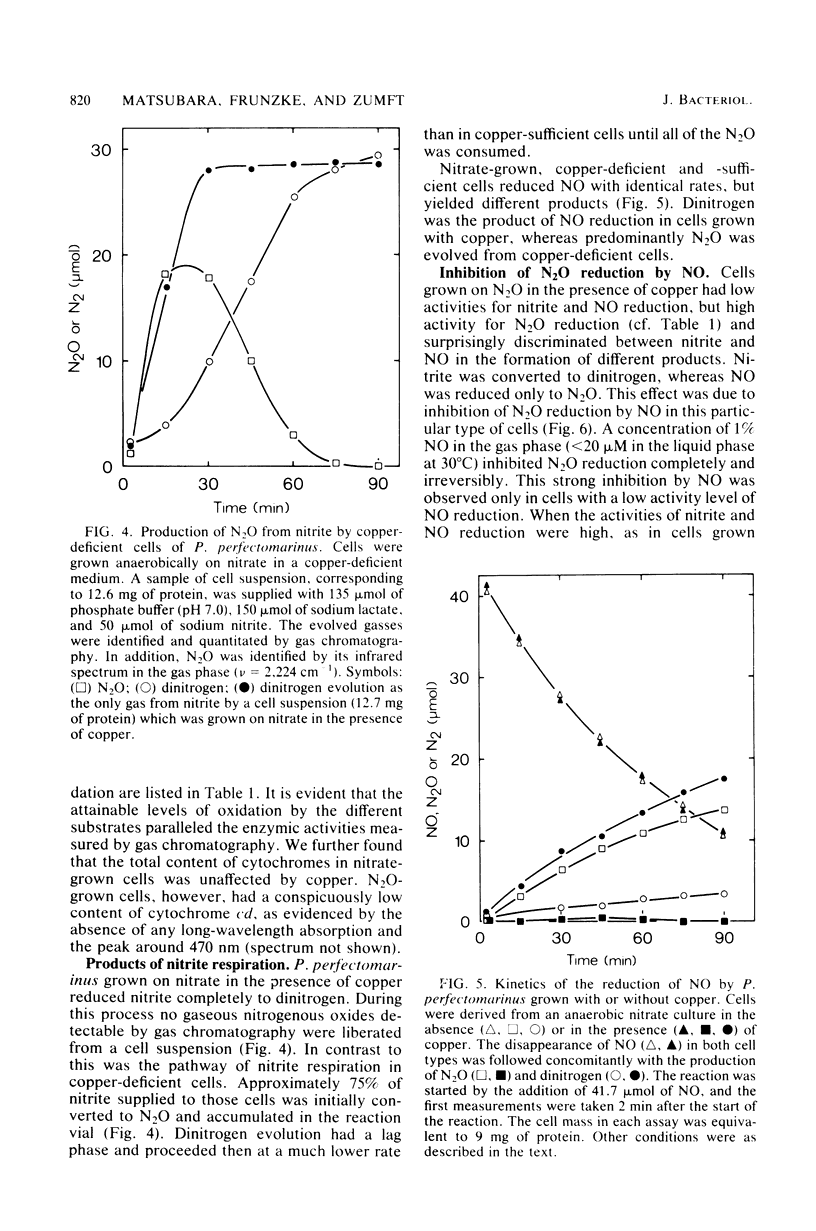
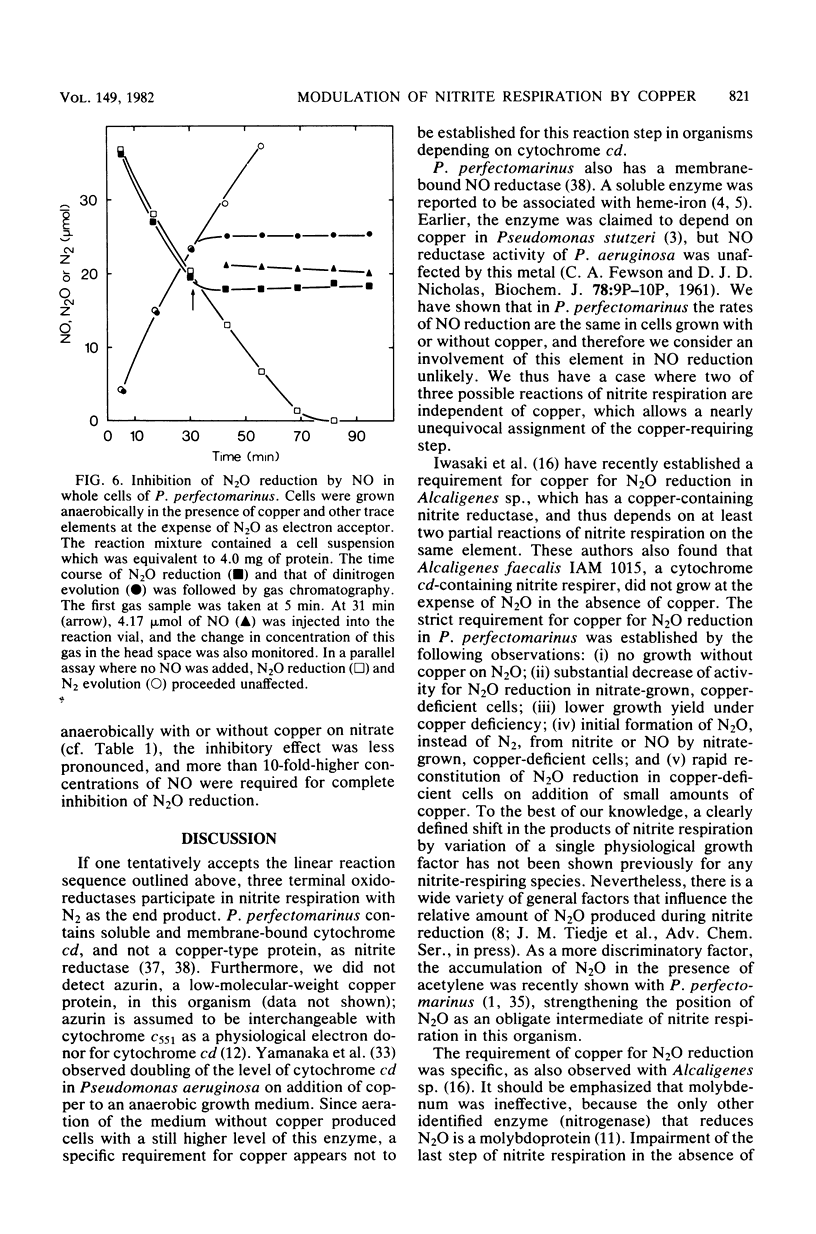
Abstract
A synthetic growth medium was purified with the chelator 1,5-diphenylthiocarbazone to study the effects of copper on partial reactions and product formation of nitrite respiration in Pseudomonas perfectomarinus. This organism grew anaerobically in a copper-deficient medium with nitrate or nitrite as the terminal electron acceptor. Copper-deficient cells had high activity for reduction of nitrate, nitrite, and nitric oxide, but little activity for nitrous oxide reduction. High rates of nitrous oxide reduction were observed only in cells grown on a copper-sufficient (1 micro M) medium. Copper-deficient cells converted nitrate or nitrite initially to nitrous oxide instead of dinitrogen, the normal end product of nitrite respiration in this organism. In agreement with this was the finding that anaerobic growth of P. perfectomarinus with nitrous oxide as the terminal electron acceptor required copper. This requirement was not satisfied by substitution of molybdenum, zinc, nickel, cobalt, or manganese for copper. Reconstitution of nitrous oxide reduction in copper-deficient cells was rapid on addition of a small amount of copper, even though protein synthesis was inhibited. The results indicate an involvement of copper protein(s) in the last step of nitrite respiration in P. perfectomarinus. In addition we found that nitric oxide, a presumed intermediate of nitrite respiration, inhibited nitrous oxide reduction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balderston W. L., Sherr B., Payne W. J. Blockage by acetylene of nitrous oxide reduction in Pseudomonas perfectomarinus. Appl Environ Microbiol. 1976 Apr;31(4):504–508. doi: 10.1128/aem.31.4.504-508.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boogerd F. C., van Verseveld H. W., Stouthamer A. H. Electron transport to nitrous oxide in Paracoccus denitrificans. FEBS Lett. 1980 May 5;113(2):279–284. doi: 10.1016/0014-5793(80)80609-0. [DOI] [PubMed] [Google Scholar]
- CHUNG C. W., NAJJAR V. A. Cofactor requirements for enzymatic denitrification. II. Nitric oxide reductase. J Biol Chem. 1956 Feb;218(2):627–632. [PubMed] [Google Scholar]
- Cox C. D., Jr, Payne W. J., Dervartanian D. V. Electron paramagnetic resonance studies on the nature of hemoproteins in nitrite and nitric oxide reduction. Biochim Biophys Acta. 1971 Nov 2;253(1):290–294. doi: 10.1016/0005-2728(71)90256-8. [DOI] [PubMed] [Google Scholar]
- Cox C. D., Jr, Payne W. J. Separation of soluble denitrifying enzymes and cytochromes from Pseudomonas perfectomarinus. Can J Microbiol. 1973 Jul;19(7):861–872. doi: 10.1139/m73-137. [DOI] [PubMed] [Google Scholar]
- Fedorova R. I., Milekhina E. I., Il'iukhina N. I. O vozmozhnosti metoda "gazoobmena" dlia obnaruzheniia zhizni vne zemli--identifikatsiia azotfiksiruiushchikh mikroorganizmov. Izv Akad Nauk SSSR Biol. 1973 Nov-Dec;6:797–806. [PubMed] [Google Scholar]
- Firestone M. K., Firestone R. B., Tiedje J. M. Nitric oxide as an intermediate in denitrification: evidence from nitrogen-13 isotope exchange. Biochem Biophys Res Commun. 1979 Nov 14;91(1):10–16. doi: 10.1016/0006-291x(79)90575-8. [DOI] [PubMed] [Google Scholar]
- Firestone M. K., Firestone R. B., Tiedje J. M. Nitrous oxide from soil denitrification: factors controlling its biological production. Science. 1980 May 16;208(4445):749–751. doi: 10.1126/science.208.4445.749. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. W., Knight E., Jr Reduction of N2O by biological N2-fixing systems. Biochem Biophys Res Commun. 1966 May 25;23(4):409–414. doi: 10.1016/0006-291x(66)90742-x. [DOI] [PubMed] [Google Scholar]
- Iwasaki H., Matsubara T. A nitrite reductase from Achromobacter cycloclastes. J Biochem. 1972 Apr;71(4):645–652. [PubMed] [Google Scholar]
- Koike I., Hattori A. Energy yield of denitrification: an estimate from growth yield in continuous cultures of Pseudomonas denitrificans under nitrate-, nitrite- and oxide-limited conditions. J Gen Microbiol. 1975 May;88(1):11–19. doi: 10.1099/00221287-88-1-11. [DOI] [PubMed] [Google Scholar]
- Kristjansson J. K., Hollocher T. C. First practical assay for soluble nitrous oxide reductase of denitrifying bacteria and a partial kinetic characterization. J Biol Chem. 1980 Jan 25;255(2):704–707. [PubMed] [Google Scholar]
- Kristjansson J. K., Walter B., Hollocher T. C. Respiration-dependent proton translocation and the transport of nitrate and nitrite in Paracoccus denitrificans and other denitrifying bacteria. Biochemistry. 1978 Nov 14;17(23):5014–5019. doi: 10.1021/bi00616a024. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsubara T., Mori T. Studies on denitrification. IX. Nitrous oxide, its production and reduction to nitrogen. J Biochem. 1968 Dec;64(6):863–871. doi: 10.1093/oxfordjournals.jbchem.a128968. [DOI] [PubMed] [Google Scholar]
- Matsubara T. Studies on denitrification. XII. Gas production from amines and nitrite. J Biochem. 1970 Feb;67(2):229–235. doi: 10.1093/oxfordjournals.jbchem.a129246. [DOI] [PubMed] [Google Scholar]
- Matsubara T. The participation of cytochromes in the reduction of N20 to N2 by a denitryfying bacterium. J Biochem. 1975 Mar;77(3):627–632. doi: 10.1093/oxfordjournals.jbchem.a130764. [DOI] [PubMed] [Google Scholar]
- Miyata M., Matsubara T., Mori T. Studies on denitrification. XI. Some properties of nitric oxide reductase. J Biochem. 1969 Dec;66(6):759–765. doi: 10.1093/oxfordjournals.jbchem.a129205. [DOI] [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J., Riley P. S., Cox C. D., Jr Separate nitrite, nitric oxide, and nitrous oxide reducing fractions from Pseudomonas perfectomarinus. J Bacteriol. 1971 May;106(2):356–361. doi: 10.1128/jb.106.2.356-361.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John R. T., Hollocher T. C. Nitrogen 15 tracer studies on the pathway of denitrification in Pseudomonas aeruginosa. J Biol Chem. 1977 Jan 10;252(1):212–218. [PubMed] [Google Scholar]
- Sørensen J., Tiedje J. M., Firestone R. B. Inhibition by sulfide of nitric and nitrous oxide reduction by denitrifying Pseudomonas fluorescens. Appl Environ Microbiol. 1980 Jan;39(1):105–108. doi: 10.1128/aem.39.1.105-108.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMANAKA T., KIJIMOTO S., OKUNUKI K. Biological significance of Pseudomonas cytochrome oxidase in Pseudomonas aeruginosa. J Biochem. 1963 May;53:416–421. doi: 10.1093/oxfordjournals.jbchem.a127716. [DOI] [PubMed] [Google Scholar]
- Yoshinari T., Knowles R. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem Biophys Res Commun. 1976 Apr 5;69(3):705–710. doi: 10.1016/0006-291x(76)90932-3. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Sherr B. F., Payne W. J. A reappraisal of the nitric oxide-binding protein of denitrifying Pseudomonas. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1230–1236. doi: 10.1016/0006-291x(79)91111-2. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Vega J. M. Reduction of nitrite to nitrous oxide by a cytoplasmic membrane fraction from the marine denitrifier Pseudomonas perfectomarinus. Biochim Biophys Acta. 1979 Dec 6;548(3):484–499. doi: 10.1016/0005-2728(79)90060-4. [DOI] [PubMed] [Google Scholar]



