Abstract
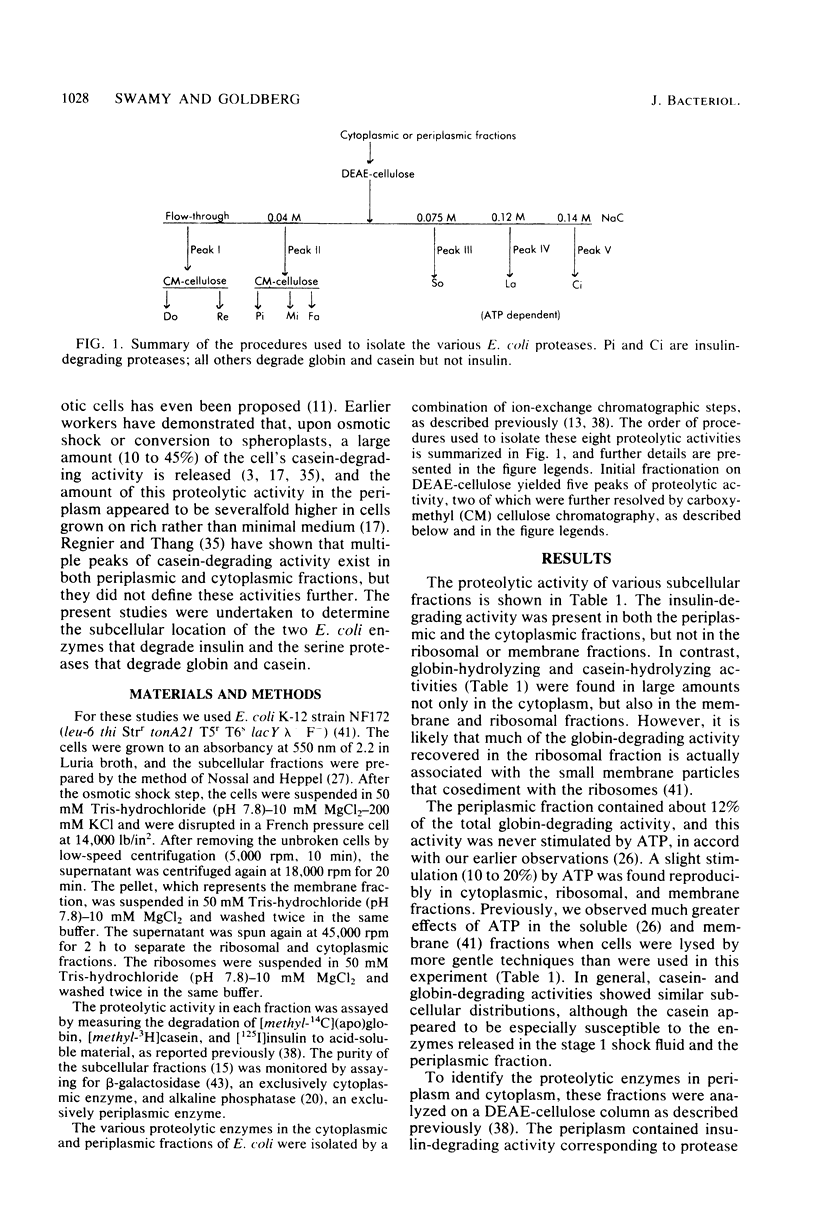
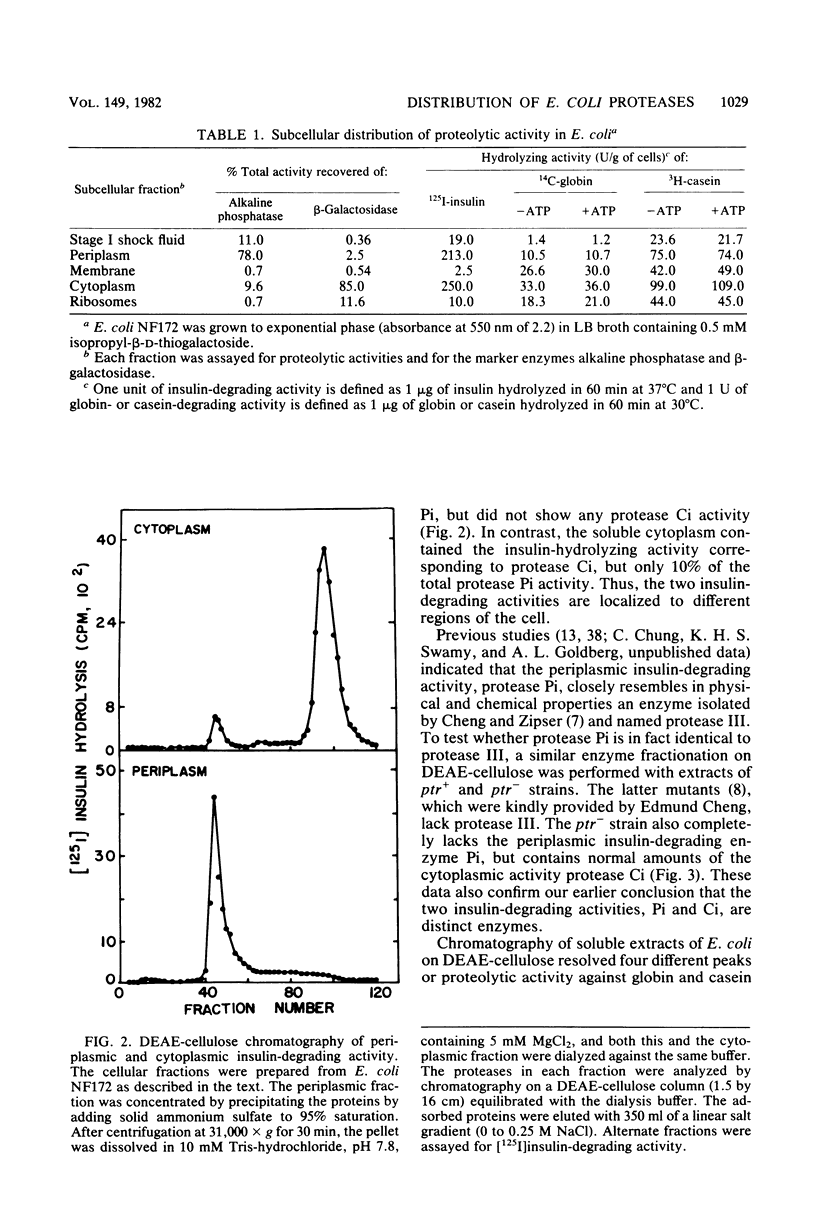
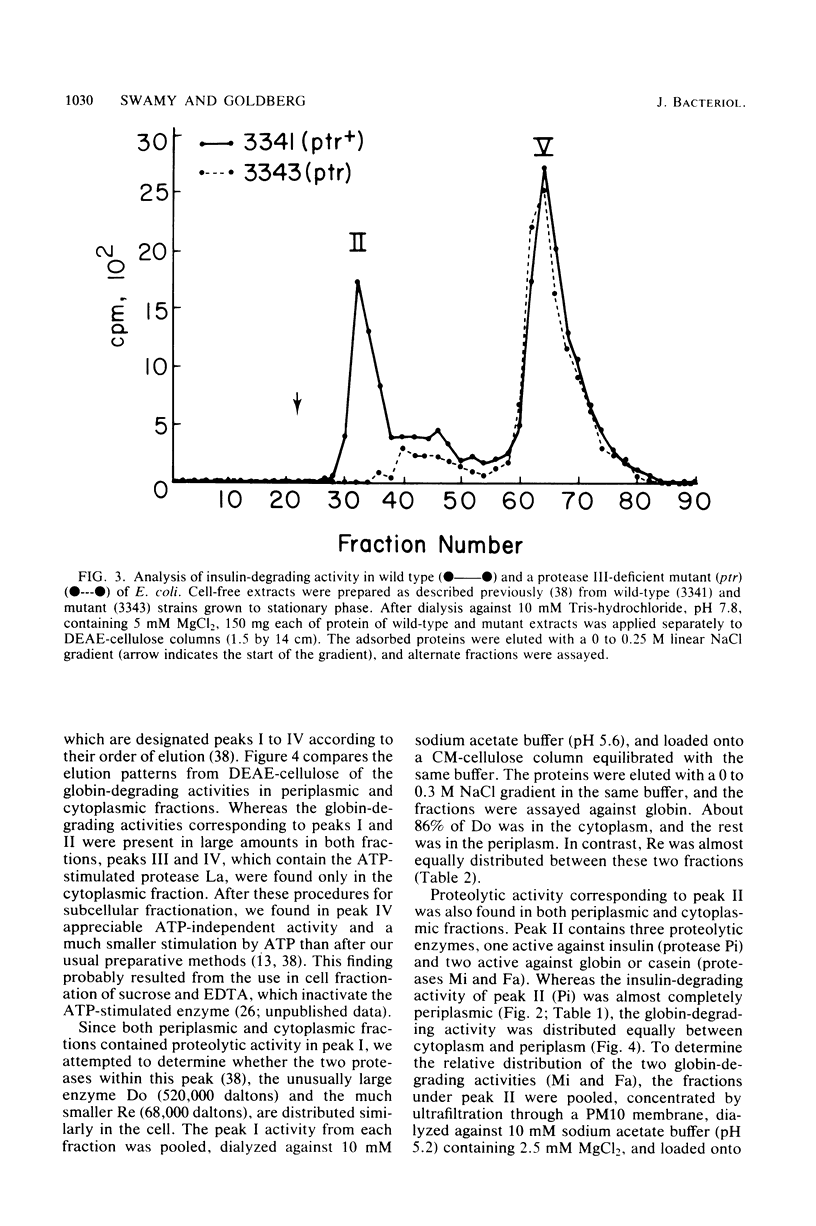
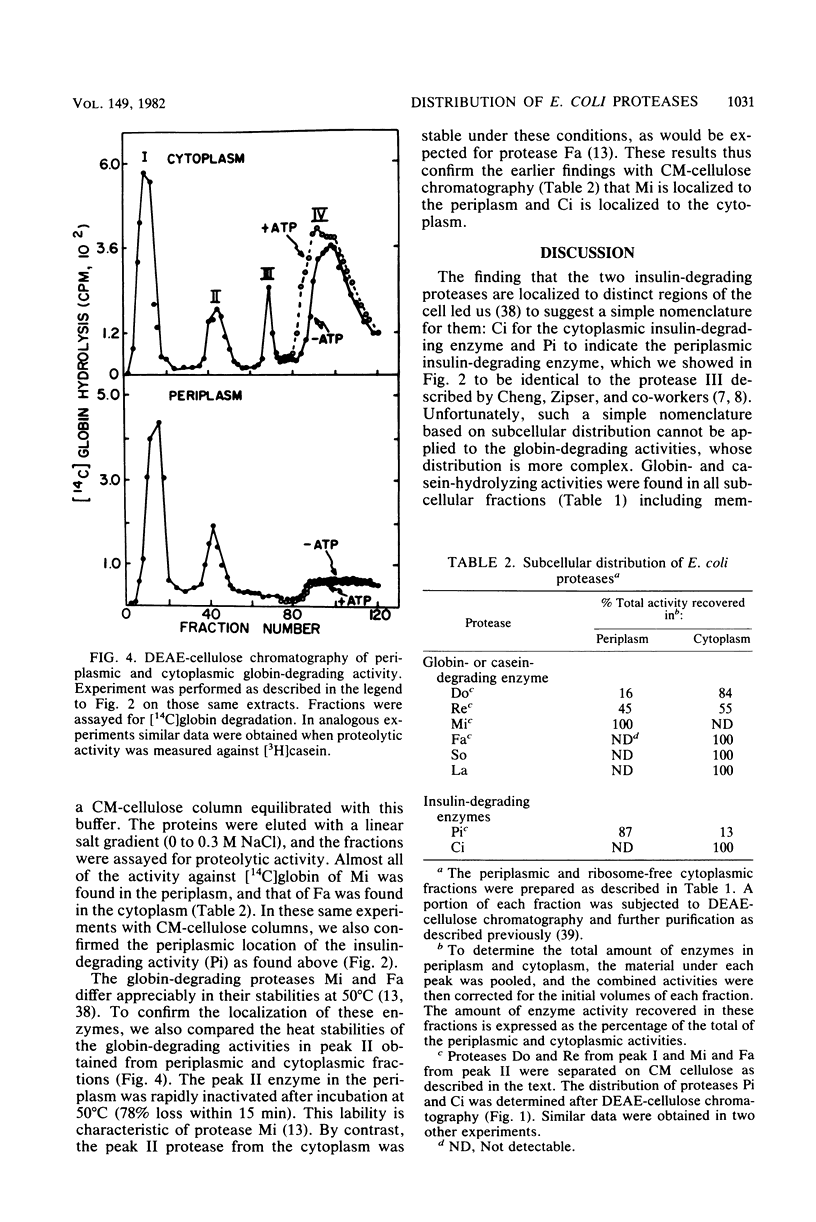
It has been reported recently that Escherichia coli cells contain eight distinct soluble enzymes capable of degrading proteins to acid-soluble material. Two are metalloproteases that degrade [125I]insulin but not larger proteins: protease Pi, which is identical to protease III, is restricted to the periplasm, and protease Ci is restriction to the cytoplasm. The six others (named Do, Re, Mi, Fa, So, and La, which is the ATP-dependent protease) are serine proteases that degrade [14C]globin and [3H]casein, but not insulin. One of these (Mi) is localized to the periplasm, and one (Re) is distributed equally between the two cellular fractions. The others are present only in the cytoplasm.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowles L. K., Konisky J. Cleavage of colicin Ia by the Escherichia coli K-12 outer membrane is not mediated by the colicin Ia receptor. J Bacteriol. 1981 Jan;145(1):668–671. doi: 10.1128/jb.145.1.668-671.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavard D., Lazdunski C. Interaction of colicin E4 with specific receptor sites mediates its cleavage into two fragments inactive towards whole cells. Eur J Biochem. 1979 Jun 1;96(3):525–533. doi: 10.1111/j.1432-1033.1979.tb13066.x. [DOI] [PubMed] [Google Scholar]
- Chang C. N., Blobel G., Model P. Detection of prokaryotic signal peptidase in an Escherichia coli membrane fraction: endoproteolytic cleavage of nascent f1 pre-coat protein. Proc Natl Acad Sci U S A. 1978 Jan;75(1):361–365. doi: 10.1073/pnas.75.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. N., Inouye H., Model P., Beckwith J. Processing of alkaline phosphatase precursor to the mature enzyme by an Escherichia coli inner membrane preparation. J Bacteriol. 1980 May;142(2):726–728. doi: 10.1128/jb.142.2.726-728.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charette M. F., Henderson G. W., Markovitz A. ATP hydrolysis-dependent protease activity of the lon (capR) protein of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4728–4732. doi: 10.1073/pnas.78.8.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Zipser D., Cheng C. Y., Rolseth S. J. Isolation and characterization of mutations in the structural gene for protease III (ptr). J Bacteriol. 1979 Oct;140(1):125–130. doi: 10.1128/jb.140.1.125-130.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Zipser D. Purification and characterization of protease III from Escherichia coli. J Biol Chem. 1979 Jun 10;254(11):4698–4706. [PubMed] [Google Scholar]
- Chung C. H., Goldberg A. L. The product of the lon (capR) gene in Escherichia coli is the ATP-dependent protease, protease La. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4931–4935. doi: 10.1073/pnas.78.8.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. D., Tai P. C. The mechanism of protein secretion across membranes. Nature. 1980 Jan 31;283(5746):433–438. doi: 10.1038/283433a0. [DOI] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Swamy K. H., Chung C. H., Larimore F. S. Proteases in Escherichia coli. Methods Enzymol. 1981;80(Pt 100):680–702. doi: 10.1016/s0076-6879(81)80052-3. [DOI] [PubMed] [Google Scholar]
- Heiman C., Miller C. G. Salmonella typhimurium mutants lacking protease II. J Bacteriol. 1978 Aug;135(2):588–594. doi: 10.1128/jb.135.2.588-594.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowit J. D., Choy W. N., Champe S. P., Goldberg A. L. Role and location of "protease I" from Escherichia coli. J Bacteriol. 1976 Dec;128(3):776–784. doi: 10.1128/jb.128.3.776-784.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowit J. D., Goldberg A. L. Intermediate steps in the degradation of a specific abnormal protein in Escherichia coli. J Biol Chem. 1977 Dec 10;252(23):8350–8357. [PubMed] [Google Scholar]
- MALAMY M., HORECKER B. L. The localization of alkaline phosphatase in E. coli K12. Biochem Biophys Res Commun. 1961 Jun 2;5:104–108. doi: 10.1016/0006-291x(61)90020-1. [DOI] [PubMed] [Google Scholar]
- MacGregor C. H., Bishop C. W., Blech J. E. Localization of proteolytic activity in the outer membrane of Escherichia coli. J Bacteriol. 1979 Jan;137(1):574–583. doi: 10.1128/jb.137.1.574-583.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel G., Wickner W. Translational and post-translational cleavage of M13 procoat protein: extracts of both the cytoplasmic and outer membranes of Escherichia coli contain leader peptidase activity. Proc Natl Acad Sci U S A. 1979 Jan;76(1):236–240. doi: 10.1073/pnas.76.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G., Heiman C., Yen C. Mutants of Salmonella typhimurium deficient in an endoprotease. J Bacteriol. 1976 Jul;127(1):490–497. doi: 10.1128/jb.127.1.490-497.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G. Peptidases and proteases of Escherichia coli and Salmonella typhimurium. Annu Rev Microbiol. 1975;29:485–504. doi: 10.1146/annurev.mi.29.100175.002413. [DOI] [PubMed] [Google Scholar]
- Miller C. G., Zipser D. Degradation of Escherichia coli beta-galactosidase fragments in protease-deficient mutants of Salmonella typhimurium. J Bacteriol. 1977 Apr;130(1):347–353. doi: 10.1128/jb.130.1.347-353.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W. The genetics of protein degradation in bacteria. Annu Rev Genet. 1980;14:279–319. doi: 10.1146/annurev.ge.14.120180.001431. [DOI] [PubMed] [Google Scholar]
- Murakami K., Voellmy R., Goldberg A. L. Protein degradation is stimulated by ATP in extracts of Escherichia coli. J Biol Chem. 1979 Sep 10;254(17):8194–8200. [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Pacaud M., Richaud C. Protease II from Escherichia coli. Purification and characterization. J Biol Chem. 1975 Oct 10;250(19):7771–7779. [PubMed] [Google Scholar]
- Pacaud M., Sibilli S., Bras G. Protease I from Escherichia coli. Some physicochemical properties and substrate specificity. Eur J Biochem. 1976 Oct 1;69(1):141–151. doi: 10.1111/j.1432-1033.1976.tb10867.x. [DOI] [PubMed] [Google Scholar]
- Pacaud M., Uriel J. Isolation and some propeties of a proteolytic enzyme from Escherichia coli (protease I). Eur J Biochem. 1971 Dec 10;23(3):435–442. doi: 10.1111/j.1432-1033.1971.tb01638.x. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Turnover of intracellular proteins. Annu Rev Microbiol. 1972;26:103–126. doi: 10.1146/annurev.mi.26.100172.000535. [DOI] [PubMed] [Google Scholar]
- Regnier P., Thang M. N. Subcellular distribution and characterization of endo and exo-cellular proteases in E. coli. Biochimie. 1972;54(10):1227–1236. doi: 10.1016/s0300-9084(72)80063-4. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L. Escherichia coli recA gene product inactivates phage lambda repressor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4714–4718. doi: 10.1073/pnas.75.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Régnier P. Identification of protease IV of E, coli. Biochem Biophys Res Commun. 1981 Apr 15;99(3):844–854. doi: 10.1016/0006-291x(81)91241-9. [DOI] [PubMed] [Google Scholar]
- Régnier P., Thang M. N. Properties of a cytoplasmic proteolytic enzyme from Escherichia coli. Eur J Biochem. 1975 Jun;54(2):445–451. doi: 10.1111/j.1432-1033.1975.tb04155.x. [DOI] [PubMed] [Google Scholar]
- Régnier P. The purification of protease IV of E. coli and the demonstration that it is an endoproteolytic enzyme. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1369–1376. doi: 10.1016/0006-291x(81)90770-1. [DOI] [PubMed] [Google Scholar]
- Strongin A. Y., Gorodetsky D. I., Stepanov V. M. The study of Escherichia coli proteases. Intracellular serine protease of E. coli-an analogue of bacillus proteases. J Gen Microbiol. 1979 Feb;110(2):443–451. doi: 10.1099/00221287-110-2-443. [DOI] [PubMed] [Google Scholar]
- Swamy K. H., Goldberg A. L. E. coli contains eight soluble proteolytic activities, one being ATP dependent. Nature. 1981 Aug 13;292(5824):652–654. doi: 10.1038/292652a0. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Guarente L., Roberts T. M., Kimelman D., Douhan J., 3rd, Ptashne M. Expression of the human fibroblast interferon gene in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5230–5233. doi: 10.1073/pnas.77.9.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voellmy R. W., Goldberg A. L. ATP-stimulated endoprotease is associated with the cell membrane of E. coli. Nature. 1981 Apr 2;290(5805):419–421. doi: 10.1038/290419a0. [DOI] [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]
- ZIPSER D. A STUDY OF THE UREA-PRODUCED SUBUNITS OF BETA-GALACTOSIDASE. J Mol Biol. 1963 Aug;7:113–121. doi: 10.1016/s0022-2836(63)80040-6. [DOI] [PubMed] [Google Scholar]
- Zwizinski C., Wickner W. Purification and characterization of leader (signal) peptidase from Escherichia coli. J Biol Chem. 1980 Aug 25;255(16):7973–7977. [PubMed] [Google Scholar]


