Abstract
We report that the small GTPase, ADP-ribosylation factor 6 (ARF6), is present only on the apical surface of polarized MDCK epithelial cells. Overexpression of a mutant of ARF6, ARF6–Q67L, which is predicted to be in the GTP-bound form, stimulates endocytosis exclusively at this surface. Surprisingly, overexpression of the mutant ARF6–T27N, which is predicted to be in the GDP-bound form, also stimulated apical endocytosis, though to a lesser extent. ARF6-stimulated endocytosis is inhibited by a dominant-negative form of dynamin, or a dominant-negative hub fragment of clathrin heavy chain, indicating that it is mediated by clathrin. Correspondingly, overexpression of either mutant of ARF6 leads to an increase in the number of clathrin-coated pits at the apical plasma membrane. When ARF6–Q67L is overexpressed in the presence of the dominant-negative dynamin, the ARF6–Q67L colocalizes with clathrin and with IgA bound to its receptor. We conclude that ARF6 is an important modulator of clathrin-mediated endocytosis at the apical surface of epithelial cells.
Keywords: receptor-mediated endocytosis, polarized Madin-Darby canine kidney cells, ADP-ribosylation factor 6, apical, clathrin
Epithelial cells have two plasma membranes (PM)1, apical (AP) and basolateral (BL), which have different compositions and perform different functions (13, 21). Clathrin-mediated endocytosis at the BL PM resembles the constitutive endocytosis found at the PM of nonpolarized cells 14. In contrast, clathrin-mediated endocytosis at the AP PM occurs at about one fifth the rate of the BL PM 14. Endocytosis at the AP PM can be stimulated in response to several intracellular signaling pathways 19, and this is essential for this PM to perform its differentiated functions of interacting with the changing external environment 13.
ADP-ribosylation factors (ARFs) are a group of small GTPases that play a central role in membrane traffic 3. The function of ARF6 has been studied by overexpression of wild-type ARF6 (ARF6–WT) and mutant forms. ARF6–Q67L is predicted to be deficient in GAP-stimulated GTP hydrolysis and thereby locked in the active GTP-bound state, while ARF6–T27N is predicted to be defective in GTP binding and is probably in a GDP-bound or nucleotide-free form. Overexpression of ARF6 mutants in fibroblasts has yielded pleiotropic effects on endocytosis, recycling, and cortical actin (5, 6, 16, 17). The localization and function of ARF6 may vary considerably, depending on the cell type. Here, we show that ARF6 regulates clathrin-mediated endocytosis at the AP PM of polarized epithelial cells.
Materials and Methods
MDCK cells were grown as described 4. Recombinant adenoviruses encoding the wild-type (WT) and mutant ARF6, as well as the clathrin hub, were produced as described 1. Levels of protein were regulated by the concentration of doxycycline (DX), amount of virus, and length of time after removal of DX. Very high levels of expression produced toxic effects. 0.1 ng/ml DX was included to partially repress ARF6 production. Cells were incubated for 16–18 h to express recombinant proteins, except clathrin hub, for 24–26 h. Unless stated, we used 60–70 pfu/cell for ARF6–WT and ARF–Q67L, or 90–100 pfu/cell for ARF6–T27N. These produced equal levels of the ARF6 proteins, as assayed by immunoblotting. For assays with 125I-IgA, 0.1 ng/ml DX was included. Using antibodies that specifically recognize the endogenous ARF6 (kind gift of V. Hsu, Harvard Medical School, Boston, MA) the level of exogenous ARF6 was approximately fivefold, relative to endogenous ARF6. For immunofluorescence, we omitted the DX to produce a ninefold overexpression relative to endogenous ARF6. These conditions minimized the possibility of toxic effects and produced an adequate signal for our localization and functional studies. Controls in all experiments included cells that were: not infected; infected but the expression of ARF6, dynamin-I K44A, or clathrin hub was fully repressed by 20 ng/ml DX; or infected with a control virus encoding β-galactosidase (gal). These caused complete loss of the ARF6, dynamin-I, or hub-specific signal in immunofluorescence and biochemical studies. Immunofluorescence was as described 11. For colocalization of ARF6 with IgA, cells were washed with cold medium and incubated with 300 μg/ml IgA for 60 min.
Processing for EM was as described 2. Cells were observed at a magnification of 19,000 and every third cell was photographed and viewed at 80 kV. A total of 30 randomly selected cell profiles were photographed. The negatives were scanned using Adobe Photoshop at a resolution of 1,000 dpi. Clathrin-coated pits were easily and reproducibly discernible. A total of 148 images were used: control, 38 images; ARF6–WT, 33 images; ARF6–Q67L, 39 images; and ARF6–T27N, 38 images. Two observers counted coated pits within each coded image and gave consistent observations. The length of the apical PM was measured using a ruler on Adobe Photoshop, and the number of each type of coated pit was divided by the length.
Endocytosis was assayed as described previously 4. For endocytosis in the presence of clathrin hub, IgA (300 μg/ml) was bound for 1 h at 4°C and cells were washed over 45 min. Cells were warmed to 37°C for 5 min. Cells were cooled to 4°C and PM-bound material was stripped with trypsin. Trypsin was neutralized with soy bean trypsin inhibitor. Cells were fixed for 40 min on ice and IgA was stained as described. Cells expressing clathrin hub were stained with T7 antibody (Novagen, Inc.).
Results and Discussion
We used a recombinant adenovirus incorporating the tetracycline transactivator system to express WT ARF6 and its mutants. Confocal microscopy showed that ARF6–WT is associated with the AP region of the cell, and not the BL region. Fig. 1 A shows a vertical X-Z section taken through the plane of a monolayer of cells expressing ARF6–WT. ARF6 staining is in green, whereas ZO-1 staining of the tight junctions is in red. Fig. 1 B shows horizontal X-Y sections taken either at the level of the AP PM (level of solid arrowhead in Fig. 1 A) or through the basal region of the cell (level of open arrowhead in Fig. 1 A). Both ARF6–WT and ARF6–Q67L were localized at, or close to, the AP PM. In contrast, ARF6–T27N gave staining in both the AP and BL regions of the cell.
Figure 1.
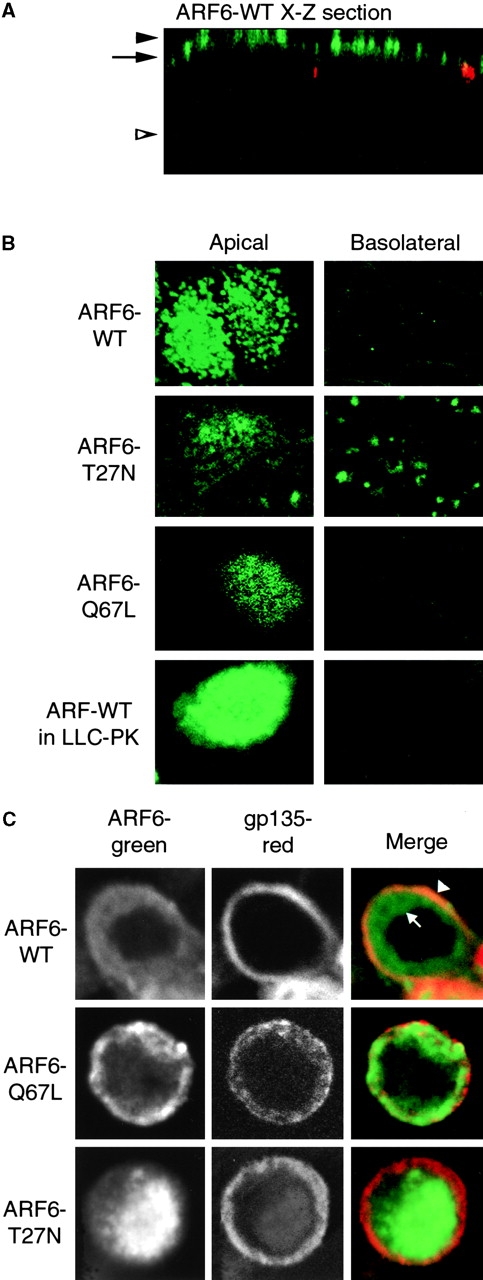
Localization of ARF6 in MDCK cells. A, X-Z section of ARF6–WT: ARF6, green (12CA5 antibody to HA epitope); Z0-1, red. B, X-Y sections taken at AP PM (level of solid arrowhead in A) or BL region (level of open arrowhead in A) of cells expressing indicated protein. ARF6–WT was also expressed in LLCPK1 cells, indicating that the AP localization of ARF6–WT is not unique to MDCK cells. C, X-Y sections taken below apical PM, at level of arrow in A.
The AP PM tends to bulge upward in our MDCK cells, forming a dome-like structure. We took advantage of this to utilize the higher X-Y confocal resolution to determine if ARF6 was entirely at the PM, or beneath the PM. Cells were costained with antibody to gp135, an endogenous AP PM protein. The X-Y sections in Fig. 1 C were just above the level of the tight junction, at the level indicated by the arrow in Fig. 1 A. In the ARF6–WT expressing cell, this X-Y section shows the gp135 staining of the AP PM as a narrow ring (Fig. 1 C). The ARF6–WT in the same cell is a broader ring. The ARF6–WT staining in the outer portion of the ring was coincident with the gp135, indicating that this portion of the ARF6–WT is at the PM (Fig. 1 C, arrowhead). The inner portion of the ARF6–WT ring is underneath the gp135 staining, i.e., in the cytoplasm beneath the AP PM (arrow). Expression of ARF6–Q67L gave a similar pattern as ARF6–WT. ARF6–T27N gave no ring, but rather, central staining that had little overlap with gp135, indicating that it is mostly absent from AP PM.
To examine the effects of ARF6 on endocytosis, we used 125I-IgA as an endocytic marker, which can be endocytosed via the polymeric immunoglobulin receptor (pIgR) at either PM. Strikingly, overexpression of either ARF6–Q67L or ARF6–T27N stimulated endocytosis of 125I-IgA from the AP PM (Fig. 2 A). ARF6–Q67L and ARF6–T27N increased endocytosis during the first minute by ∼3-fold or 1.5-fold, respectively. In contrast, AP endocytosis of IgA in cells overexpressing ARF6–WT did not markedly differ from control cells infected with a virus encoding β-gal or uninfected cells. Overexpression of ARF6–WT or either mutant did not significantly alter the rate or extent of BL endocytosis, recycling back to the AP PM, BL to AP transcytosis, or BL to BL recycling (our unpublished data). Therefore, only AP endocytosis was affected by ARF6.
Figure 2.
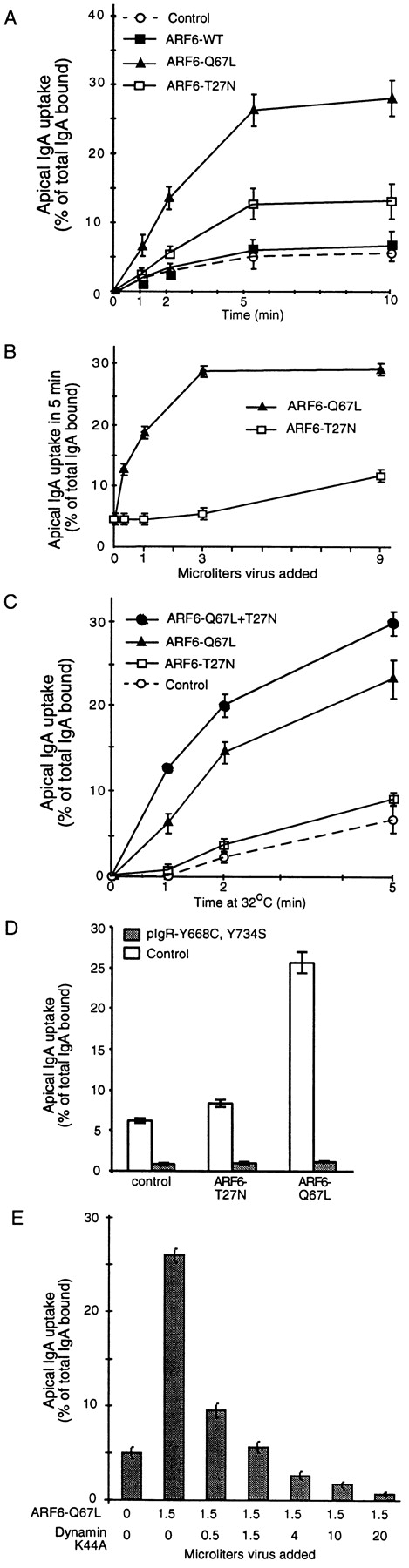
Effect of ARF6 on AP endocytosis. A, Effect on rate of endocytosis. B, Effects of varying expression level of ARF6 mutants on AP endocytosis. Cells were infected with the indicated quantity of virus. Endocytosis was assayed for 5 min at 37°C, 1 μl of virus ∼25 pfu/cell. C, Effect of coexpression of ARF6 mutants. Cells were either infected with virus for ARF6–Q67L plus virus for β-gal (to keep the total virus constant), ARF6–T27N plus β-gal virus, or doubly infected with viruses encoding both ARF6 mutants. To improve temporal resolution, this experiment was conducted at 32°C. D, Effect of ARF6 on endocytosis of endocytosis-deficient mutant pIgR. Endocytosis of pIgR with both cytoplasmic Tyr mutated to Ala was assayed in control cells or cells expressing ARF6–WT or mutants. In this experiment only, normal MDCK cells stably expressing the pIgR-664A, 734A, or wild-type pIgR were coinfected with the virus for β-gal or ARF6 mutant, plus 70–80 pfu/cell of adenovirus encoding the transactivator. The level of expression of the ARF6 mutant proteins was comparable to other experiments. E, Cells were doubly infected with ARF6–Q67L and the indicated quantity of virus for dynamin-I K44A. Endocytosis over 5 min was measured. Expression of dynamin did not alter expression of the ARF6, as assayed by immunoblot (not shown).
In Fig. 2 B, levels of ARF6–Q67L or ARF6–T27N were manipulated by infecting cells with varying amounts of virus. The stimulatory effect of ARF6–Q67L was significantly greater than that of ARF6–T27N at all expression levels examined. In cells simultaneously expressing ARF6–Q67L and ARF6–T27N (achieved by double infection), we observed increased stimulation of endocytosis above levels induced by ARF6–Q67L alone (Fig. 2 C).
We next tested if ARF6 could stimulate endocytosis of markers that are not internalized via clathrin-coated pits. Mutation of both cytoplasmic Tyr in pIgR gave a pIgR that is endocytosed at about five percent of the rate of the WT 15. Fig. 2 D shows that neither ARF6–Q67L nor ARF6–T27N stimulated endocytosis of this mutant. ARF6 did not affect AP internalization of ricin or fluid phase markers (our unpublished data), confirming that ARF6 does not affect nonclathrin endocytosis.
To test if ARF6–Q67L stimulates clathrin-mediated endocytosis, we inhibited clathrin function by cytosol acidification or hypertonic media, and found that ARF6–Q67L-stimulated endocytosis was blocked (our unpublished results). We next tested the ability of the K44A mutant of tge dynamin-I mutant to inhibit ARF6–Q67L-stimulated endocytosis 1. Coinfection with virus encoding dynamin-I K44A inhibited the endocytosis stimulated by ARF6–Q67L, and this inhibition exhibited a dependence on the amount of dynamin-I K44A expressed (Fig. 2 E). Therefore, endocytosis stimulated by ARF6–Q67L is dynamin-dependent.
Overexpression of a fragment of clathrin heavy chain, clathrin hub, acts as a dominant-negative inhibitor of clathrin-mediated endocytosis 10. We made recombinant virus expressing the hub, and coexpressing both the clathrin hub and ARF6 (WT or mutants). Uptake of IgA was analyzed by immunofluorescence microscopy, using the T7 epitope tag on clathrin hub to detect transfected cells. We found an inverse correlation between expression of clathrin hub and internalization of IgA (Fig. 3). The inhibition of AP IgA endocytosis by the clathrin hub was true for cells not overexpressing ARF6, as well as cells expressing ARF6–WT and the two mutants (Fig. 3). Therefore, AP endocytosis of IgA promoted by ARF6 mutants is clathrin-mediated.
Figure 3.
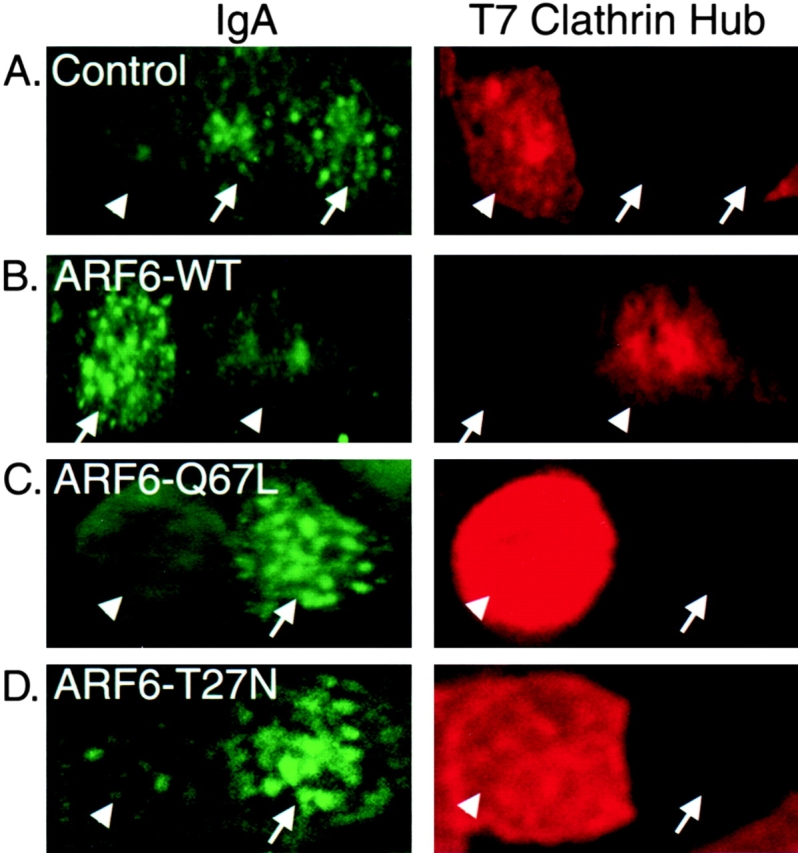
Clathrin hub inhibits ARF6 stimulated AP endocytosis. Cells were infected with 150 pfu/cell of virus expressing the dominant-negative clathrin hub alone, or also with virus for ARF6–WT or mutants. Cells were incubated with IgA at the AP PM for 60 min at 4°C, then at 37°C for 5 min. IgA remaining on the PM was stripped with trypsin at 4°C, under conditions that remove >99% of PM-bound IgA. After fixation and permeabilization, IgA was stained with antibodies to human IgA, while the clathrin hub was stained with an antibody to its T7 epitope tag. Arrowheads indicate cells expressing a high level of hub, which do not endocytose IgA. Arrows indicate cells in which clathrin hub was not detected; these cells do endocytose IgA. All cells expressed pIgR on their AP PM.
As ARF6-stimulated endocytosis is clathrin-mediated, we asked if ARF6 overexpression recruited clathrin to the AP PM. Confocal sections were taken at the level indicated by the solid arrowhead in Fig. 1 A. Cells expressing ARF6–WT have a small increase in AP PM clathrin (Fig. 4 A). Overexpression of either ARF6–Q67L or ARF6–T27N gave a greater increase in clathrin.
Figure 4.
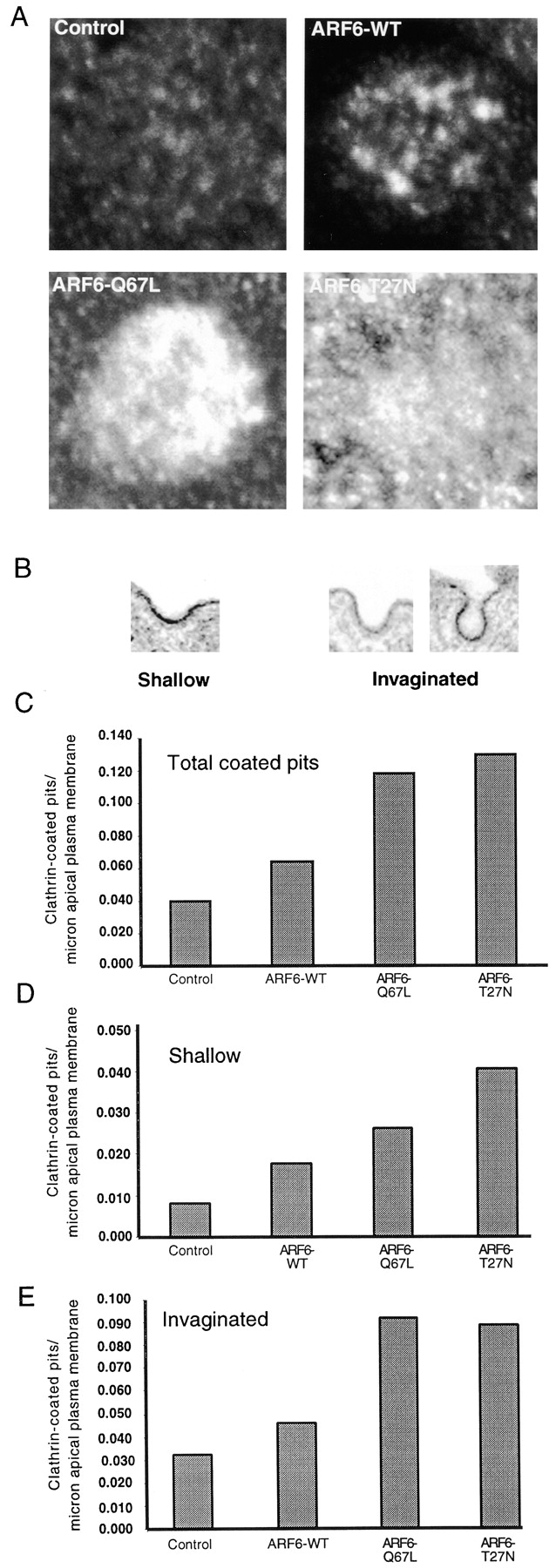
ARF6 recruits clathrin to the AP PM. A, Cells were either uninfected or infected with adenovirus for ARF6–WT or mutants, then stained for clathrin. Confocal sections are at the level of the arrowhead in Fig. 1 A. B, Examples of shallow and invaginated clathrin-coated pits, as seen by EM. C, Total coated pits/μm AP PM. D, Shallow coated pits. E, Invaginated coated pits.
We next asked if the number or morphology of clathrin-coated pits was altered, using thin-section EM under conditions where clathrin coats were easily identified. We classified clathrin-coated pit profiles as shallow (width of pit > depth), or invaginated (depth ≥ width). Examples are shown in Fig. 4 B. Fig. 4 C shows that overexpression of ARF6–WT gave a modest increase in coated pits, as compared with control. This is in agreement with the small increase in clathrin recruitment seen in Fig. 4 A. Consistent with this, we found that overexpression of ARF6–WT at a higher level than we routinely used stimulated AP endocytosis somewhat (data not shown). Overexpression of either ARF6–Q67L or ARF6–T27N gave a much greater increase of coated pit profiles. Notably, the greatest number of shallow pits was found with ARF6–T27N (Fig. 4 D; this finding may have particular significance). In contrast, ARF6–Q67L and ARF6–T27N gave nearly equal numbers of invaginated pits (Fig. 4 E). These results further support the hypothesis that ARF6 affects clathrin-mediated endocytosis at the AP PM.
We next investigated if ARF6 is present, even transiently, in coated pits by trapping ARF6 in the deeply invaginated coated pits induced by dynamin-I K44A 9. As shown in Fig. 5, there was significant colocalization of ARF6–WT and ARF6–Q67L with endogenous clathrin at the AP PM of cells expressing dynamin-I K44A. This indicates that ARF6 may normally cycle through coated pits, and when coated pit pinching-off is prevented, a significant fraction of the ARF6–WT or ARF6–Q67L can be trapped.
Figure 5.
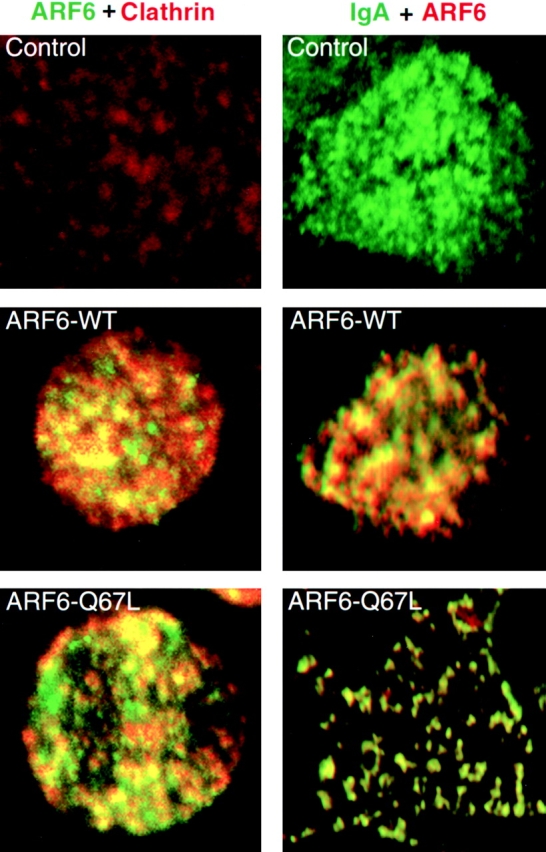
Colocalization of ARF6, clathrin, and IgA in cells expressing dynamin-I K44A. Left, Cells were infected with viruses for dynamin-I K44A (100 pfu/cell), and either ARF6–WT or ARF6–Q67L. Cells were treated for 5 min at 4°C with 120 μg/ml digitonin 10 to extract cytoplasmic clathrin, washed three times with cold PBS, fixed, and processed for immunofluorescence to detect ARF6 and endogenous clathrin. Right, Cells were infected with viruses for pIgR, dynamin-I K44A, and either β-gal, ARF6–WT, or ARF6–Q67L. IgA was bound to the AP PM for 1 h at 4°C. Cells were warmed to 15°C for 10 min. Unpermeabilized cells were stained for IgA, then cells were extracted with digitonin, fixed, permeabilized, and ARF6 stained.
We also investigated if IgA bound to pIgR at the AP PM would accumulate in clathrin-coated pits under these conditions. Ordinarily, although endocytic receptors such as pIgR and TfR are concentrated 5–10-fold in clathrin-coated pits, ∼80–90% of these receptors are at steady state located outside of coated pits. Fig. 5 shows that ARF6–WT and ARF6–Q67L significantly colocalize with IgA under these conditions.
How does ARF6 influence AP endocytosis? Like other small GTPases, ARF6 probably has multiple effectors, and these might be responsible for the cell type-specific differences in ARF6 function 12. ARF6 also acts on the cortical actin cytoskeleton (18, 20). The actin cytoskeleton underlying the AP PM of epithelial cells has a unique organization, involving actin cores in microvilli and an underlying terminal web. Cytochalasin D inhibits clathrin-mediated endocytosis at the AP PM of MDCK cells 8. ARF6 may act on the AP actin cytoskeleton to regulate clathrin-mediated AP endocytosis.
Both ARF6–Q67L and ARF6–T27N stimulate AP endocytosis, and coexpression of the two proteins produces an even greater stimulation. In macrophages, both ARF6–Q67L and ARF6–T27N inhibit phagocytosis, providing a precedent for both mutant forms of ARF6, giving effects in the same direction 23. Although both ARF6–Q67L and ARF6–T27N stimulate endocytosis, there are quantitative and qualitative differences in their effects, suggesting differences in their modes of action. ARF6–Q67L is more potent in stimulating endocytosis. Although both ARF6–Q67L and ARF6–T27N give equal increases in the number of clathrin-coated pits, ARF6–T27N gives a greater increase in shallow pits (Fig. 4). This suggests that ARF6–T27N may preferentially increase the formation of shallow pits and/or decrease the rate of progression to invaginated pits. In contrast, the greater increase in endocytosis produced by ARF6–Q67L suggests that it acts on a later step in endocytosis. ARF6–WT and ARF6–Q67L can be trapped in clathrin-coated pits when pinching-off of the pits is prevented. ARF6 may normally cycle through coated pits, but then leaves the pits so rapidly that an accumulation in coated pits is not ordinarily detectable. The large stimulation of endocytosis caused by ARF6–Q67L could be due to a direct action on coated pits themselves. In contrast, ARF6–T27N is largely not on the AP PM. Its modest stimulation of AP endocytosis may be due to an indirect effect, e.g., via the actin cytoskeleton. Further work will be needed to distinguish this model from other possibilities.
Our results illustrate the importance of comparing the localization and function of proteins in nonpolarized and polarized cells. ARF6 may regulate a specialized endocytic pathway in nonpolarized cells that is cognate to AP endocytosis in epithelial cells (7, 17, 22). As cells develop a polarized AP PM, ARF6 and the specialized endocytic pathway may become restricted to that PM.
Acknowledgments
We thank L. O'Brien, R. Kelly, S. Schmid, and H. Bourne for discussions, and V. Hsu, G. Ojakian, and S. Schmid for reagents.
Supported by DAMD17-1-97-1-7326 to Y. Altshuler, DK51970-01 to G. Apodaca, GM38093 and GM57657 to F. Brodsky, and National Institutes of Health grants to K. Mostov.
Footnotes
1.used in this paper: AP, apical; ARF6, ADP-ribosylation factor 6; ARF6–T27N, mutant ARF6 predicted to be in the GDP-bound form; ARF6–Q67L, mutant ARF6 predicted to be in the GTP-bound form; ARF6–WT, wild-type ARF6; BL, basolateral; DX, doxycycline; gal, galactosidase; pIgR, polymeric immunoglobulin receptor; PM, plasma membrane; WT, wild-type
References
- Altschuler Y., Barbas S.M., Terlecky L.J., Tang K., Hardy S., Mostov K.E., Schmid S.L. Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms. J. Cell Biol. 1998;143:1871–1881. doi: 10.1083/jcb.143.7.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G., Aroeti B., Tang K., Mostov K.E. Brefeldin-A inhibits the delivery of the polymeric immunoglobulin receptor to the basolateral surface of MDCK cells. J. Biol. Chem. 1993;268:20380–20385. [PubMed] [Google Scholar]
- Boman A.L., Kahn R.A. Arf proteinsthe membrane traffic police? Trends Biochem. Sci. 1995;20:147–150. doi: 10.1016/s0968-0004(00)88991-4. [DOI] [PubMed] [Google Scholar]
- Breitfeld P.P., Harris J.M., Mostov K.M. Postendocytotic sorting of the ligand for the polymeric immunoglobulin receptor in Madin-Darby canine kidney cells. J. Cell Biol. 1989;109:475–486. doi: 10.1083/jcb.109.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenaugh M.M., Whitney J.A., Carroll K., Zhang C.-J., Boman A.L., Rosenwald A.G., Mellman I., Kahn R.A. Intracellular distribution of Arf proteins in mammalian cells. J. Biol. Chem. 1996;271:21767–21774. doi: 10.1074/jbc.271.36.21767. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Li G., Colombo M.I., Stahl P.D. A regulatory role for ARF6 in receptor-mediated endocytosis. Science. 1995;267:1175–1178. doi: 10.1126/science.7855600. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C., van Donselaar E., Hsu V.W., Yang C., Stahl P.D., Peters P.J. ARF6 targets recycling vesicles to the plasma membraneinsights from an ultrastructural investigation. J. Cell Biol. 1998;140:603–616. doi: 10.1083/jcb.140.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb T.A., Ivanov I.E., Adesnik M., Sabatini D.D. Actin microfilaments play a critical role in endocytosis at the apical, but not the basolateral surface of polarized epithelial cells. J. Cell Biol. 1993;120:695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte S.A., Oakley R.H., Zhang J., Holt J.A., Ferguson S.S.G., Caron M.G., Barak L.S. The 2-adrenergic receptor/arrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc. Natl. Acad. Sci. USA. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.H., Marks M.S., Brodsky F.M. A dominant-negative clathrin mutant differentially affects trafficking of molecules with distinct sorting motifs in the class II major histocompatibility complex (MHC) pathway. J. Cell Biol. 1998;140:1023–1037. doi: 10.1083/jcb.140.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low S.-H., Chapin S.J., Weimbs T., Kömüves L.G., Bennett M.K., Mostov K.E. Differential localization of syntaxin isoforms in polarized MDCK cells. Mol. Biol. Cell. 1996;7:2007–2018. doi: 10.1091/mbc.7.12.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massenburg D., Han J.-S., Liyanage M., Patton W.A., Rhee S.G., Moss J., Vaughan M. Activation of rat brain phospholipase D by ADP-ribosylation factors 1, 5, and 6separation of ADP-ribosylation factor-dependent and oleate-dependent enzymes. Proc. Natl. Acad. Sci. USA. 1994;91:11718–11722. doi: 10.1073/pnas.91.24.11718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K.E., Cardone M.H. Regulation of protein traffic in polarized epithelial cells. BioEssays. 1995;17:129–138. doi: 10.1002/bies.950170208. [DOI] [PubMed] [Google Scholar]
- Naim H.Y., Dodds D.T., Brewer C.B., Roth M.G. Apical and basolateral coated pits of MDCK cells differ in their rates of maturation into coated vesicles, but not in the ability to distinguish between mutant hemagglutinin proteins with different internalization signals. J. Cell Biol. 1995;129:1241–1250. doi: 10.1083/jcb.129.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto C.T., Shia S.-P., Bird C., Mostov K.E., Roth M.G. The cytoplasmic domain of the polymeric immunoglobulin receptor contains two internalization signals that are distinct from its basolateral sorting signal. J. Biol. Chem. 1992;267:9925–9932. [PubMed] [Google Scholar]
- Peters P.J., Hsu V.W., Ooi C.E., Finazzi D., Teal S.B., Oorschot V., Donaldson J.G., Klausner R.D. Overexpression of wild-type and mutant ARF1 and ARF6distinct perturbations of nonoverlapping membrane compartments. J. Cell Biol. 1995;128:1003–1017. doi: 10.1083/jcb.128.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H., Donaldson J.G. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J. Cell Biol. 1997;139:49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H., Klausner R.D., Donaldson J.G. Aluminum fluoride stimulates surface protrusions in cells overexpressing the ARF6 GTPase. J. Cell Biol. 1996;134:935–947. doi: 10.1083/jcb.134.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., van Deurs B. Endocytosis, intracellular transport, and cytotoxic action of Shiga toxin and ricin. Physiol. Rev. 1996;76:949–966. doi: 10.1152/physrev.1996.76.4.949. [DOI] [PubMed] [Google Scholar]
- Song J., Khachikian Z., Radhakrishna H., Donaldson J.G. Localization of endogenous ARF6 to sites of cortical actin rearrangement and involvement of ARF6 in cell spreading. J. Cell Sci. 1998;111:2257–2267. doi: 10.1242/jcs.111.15.2257. [DOI] [PubMed] [Google Scholar]
- Weimbs T., Low S.-H., Chapin S.J., Mostov K.E. Apical targeting in polarized epithelial cellsthere's more afloat than rafts. Trends Cell Biol. 1997;7:393–399. doi: 10.1016/S0962-8924(97)01130-6. [DOI] [PubMed] [Google Scholar]
- Wilson J.M., Colton T.L. Targeting of an intestinal apical endosomal protein to endosomes in nonpolarized cells. J. Cell Biol. 1997;136:319–330. doi: 10.1083/jcb.136.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Cox D., Tseng C.C., Donaldson J.G., Greenberg S. A requirement for ARF6 in Fcγ receptor-mediated phagocytosis in macrophages. J. Biol. Chem. 1998;273:19977–19981. doi: 10.1074/jbc.273.32.19977. [DOI] [PubMed] [Google Scholar]


