Abstract
Signaling by members of the epidermal growth factor receptor family plays an important role in breast development and breast cancer. Earlier work suggested that one of these receptors, ErbB4, is coupled to unique responses in this tissue. To determine the function of ErbB4 signaling in the normal mouse mammary gland, we inactivated ErbB4 signaling by expressing a COOH terminally deleted dominant-negative allele of ErbB4 (ErbB4ΔIC) as a transgene in the mammary gland. Despite the expression of ErbB4ΔIC from puberty through later stages of mammary development, an ErbB4ΔIC-specific phenotype was not observed until mid-lactation. At 12-d postpartum, lobuloalveoli expressing ErbB4ΔIC protein were condensed and lacked normal lumenal lactation products. In these lobuloalveoli, β-casein mRNA, detected by in situ hybridization, was normal. However, whey acidic protein mRNA was reduced, and α-lactalbumin mRNA was undetectable. Stat5 expression was detected by immunohistochemistry in ErbB4ΔIC-expressing tissue. However, Stat5 was not phosphorylated at Y694 and was, therefore, probably inactive. When expressed transiently in 293T cells, ErbB4 induced phosphorylation of Stat5. This phosphorylation required an intact Stat5 SH2 domain. In summary, our results demonstrate that ErbB4 signaling is necessary for mammary terminal differentiation and Stat5 activation at mid-lactation.
Keywords: ErbB4, Stat5, dominant-negative mutant, transgenic mice, mammary gland development
Aberrant signaling activity by members of the epidermal growth factor receptor (EGFR)1 family of tyrosine kinases frequently occurs in human cancer. The family consists of the EGFR (HER), ErbB2 (HER2/Neu), ErbB3 (HER3), and ErbB4 (HER4). These receptors are activated by binding of growth factors in the EGFR family, which are encoded by at least nine genes. The ligand-activated receptors can signal either through homodimerization or through heterodimerization with other EGFR family members. Each receptor/ligand combination has the potential to recruit and activate a unique set of interacting proteins, thereby initiating signaling cascades which culminate in distinct cellular responses (reviewed in Alroy and Yarden 1997; Riese and Stern 1998).
Overexpression of three of these receptors, EGFR, ErbB2, and ErbB3, is associated with carcinogenesis. For example, amplification of EGFR and ErbB2, or both, occurs in a large subset of breast carcinomas, and is associated with poor prognosis or the presence of negative prognostic indicators (reviewed in Hynes and Stern 1994; Dickson and Lippman 1995). Elevated levels of ErbB3 have been reported in 22–55% of breast carcinomas (Lemoine et al. 1992; Quinn et al. 1994; Bobrow et al. 1997; Naidu et al. 1998). In contrast, ErbB4 is normally found in differentiating tissues (Srinivasan et al. 1998), ErbB4 overexpression in breast cancer is associated with favorable prognostic factors (Bacus et al. 1994, Bacus et al. 1996; Knowlden et al. 1998), and ErbB4 expression may be selectively extinguished during breast tumor progression (Srinivasan et al. 1998). These results suggest that ErbB4 is coupled to a differentiation response, rather than the proliferation response associated with the other three EGFR family members in mammary epithelium.
The role of ErbB4 in mammary differentiation is further supported by patterns of receptor expression and activation during normal mouse mammary gland development. Unlike EGFR and ErbB2, which are expressed and activated during puberty (Schroeder and Lee 1998; Sebastian et al. 1998), ErbB4 expression is first strong in mature females (Schroeder and Lee 1998). Although ErbB4 is expressed strongly throughout pregnancy, ErbB4 tyrosine phosphorylation, an indicator of active signaling, was only first detected at late pregnancy, when epithelial differentiation and secretory activity predominate over proliferation, and peaked during lactation, when mammary epithelium undergo terminal differentiation (Schroeder and Lee 1998). Moreover, we previously demonstrated that the ErbB3/ErbB4 ligand neuregulin-1 (NRG1), but not the EGFR ligand transforming growth factor α (TGFα), induces epithelial differentiation, with expression of the milk protein β-casein, when implanted in mammary glands of virgin mice (Jones et al. 1996). Finally, a role for NRG1 in mammary differentiation is consistent with high levels of NRG1 expression observed during pregnancy (Yang et al. 1995).
Stat5, a member of the signal transducers and activators of transcription (Stat) family, also appears to play an important role in mammary differentiation. Stat5 was initially identified as mammary gland factor in nuclear extracts from lactating mice (Wakao et al. 1992, Wakao et al. 1994). Expression, activation by phosphorylation, and nuclear translocation of Stat5 are tightly linked to mammary differentiation (Kazansky et al. 1995; Liu et al. 1996a). Consistent with these results, both Stat5a and Stat5b are required for normal milk gene expression and lactation at parturition (Liu et al. 1996b; Teglund et al. 1998). Although Stat5 was first identified as a prolactin-regulated transducer, members of the EGF family of growth factors recently have been shown to activate Stat5 through tyrosine phosphorylation of the Stat5 polypeptide (Ruff-Jamison et al. 1995; David et al. 1996; Garcia et al. 1997; Richer et al. 1998; Olayioye et al. 1999). These latter results raise the possibility that Stat5 can be activated by EGFR family members during mammary differentiation.
Although the timing of expression of ErbB4 ligands and activation of ErbB4 suggest that ErbB4 regulates differentiation, the function of ErbB4 signaling during mammary gland development is not known. To identify the role of ErbB4 signaling during breast development, we inactivated endogenous ErbB4 signaling in the mouse mammary gland through transgenic expression of a mutant ErbB4 with dominant-negative activity. The results identify a role for ErbB4 in terminal differentiation of mammary epithelium, including activation of the important mediator of mammary differentiation, Stat5.
Materials and Methods
Plasmids
pMMTV-ErbB4ΔIC contains a truncated human ErbB4 cDNA in which sequences encoding ErbB4 up to P705 are fused to sequences encoding tandem Flag epitope tags (Kodak), immediately followed by two stop codons. The coding sequences are under regulatory control of a mouse mammary tumor virus (MMTV) long terminal repeat. The vector pMMTV-SV40-Bssk (pMMTV-GAL4/236-SV40 minus the GAL4/236 gene; Ornitz et al. 1991; generously provided by Dr. Philip Leder, Harvard Medical School, Boston, MA) was first modified to generate the vector pMMTV-Aat using the AatII linker 5′-ACTAGTGACGTCA at the unique SpeI site. pMMTV-ErbB4ΔIC was produced by trimolecular ligation joining the HindIII (site filled with T4 DNA polymerase)-EcoRI digested pMMTV-Aat; the 3′ ∼2.1 Kb SalI (site filled with T4 DNA polymerase)-SpeI fragment from pLXSN-ErbB4 (Riese et al. 1995); and the 285-bp SpeI-EcoRI digested PCR product generated from pLXSN-ErbB4 using the forward primer upstream of the unique SpeI site 5′-CCCACTAGTCATGAC (nt 1905–1919; Plowman et al. 1993) and the reverse primer with an EcoRI linker 5′-CGGAATTCTTATTACTTGTCATCGTCGTCTTGTAGTCCTTGTCATCGTCGTCCTTGTAGTCGGGTG-CTGTGCC-3′ (nt 2138–2127; Plowman et al. 1993).
The riboprobe template pBl-ErbB4ΔIC was produced by subcloning the 285-bp SpeI-EcoRI digested PCR product described above into pBluescript I S/K (Stratagene). Riboprobe template pBl-β-casein was generated by subcloning the 170-bp HindIII-StuI fragment from pFLAG1-β-casein (generously supplied by Dr. Nancy Hynes, Friedrich Miescher Institute, Basel, Switzerland) into HindIII-SmaI digested pBluescript I S/K. The riboprobe template pBl-WAP, for quantifying mouse whey acidic protein (WAP) RNA, was generated by reverse transcriptase PCR (RT-PCR) of mouse mammary gland RNA isolated from a female at 3-d postpartum using the downstream primer 5′-CTATCTGCATTGGGCACGGCCCGG (nt 319–296; Hennighausen and Sippel 1982). The cDNA was amplified by PCR using the downstream primer specified above, and the upstream primer 5′-CCTCATCAGCCTTGTTCTTGGCCT (nt 24–47; Hennighausen and Sippel 1982). The 295-bp PCR product was made blunt with T4 DNA polymerase and subcloned into the SmaI site of pBluescript I S/K. The riboprobe template pBl-α-lactalbumin, was generated by RT-PCR with the downstream primer 5′-GGGCTTCTCACAACGCCACTGTTC (nt 439–416; Vilotte and Soulier 1992) and the upstream primer 5′-CATAGATGGCTATCAAGGCATCTC (nt 114–137; Vilotte and Soulier 1992). The PCR product was digested with HincII-HindIII and the resultant 214-bp fragment was subcloned into pBluescript I K/S.
Generation of MMTV-ErbB4ΔIC Transgenic Mice
Vector sequences for microinjection were separated from pMMTV-ErbB4ΔIC by digestion at unique AatII-XhoI sites. The ∼6.2-kb fragment containing the MMTV LTR, a 600-bp 5′ untranslated region of c-Ha-ras, the truncated human ErbB4 cDNA with COOH-terminal tandem Flag epitope tags, and simian virus 40 3′ mRNA processing signals, was purified by agarose gel electrophoresis. The purified DNA fragment was microinjected into single-cell B6SJL/F2 zygotes at a concentration of 12 μg/ml in 10 mM Tris, pH 7.5, 0.1 mM EDTA (by Ms. Carole Pelletier under the direction of Dr. David Brownstein at the Transgenic Mouse Shared Resource of the Yale University School of Medicine, New Haven, CT).
Identification of Transgenic Mice by PCR
Transgenic mice were identified by PCR of DNA isolated from tail biopsies. DNA purification, PCR conditions, and controls have been described previously (Jones and Stern 1999). Primers for the amplification of a 360-bp MMTV-ErbB4ΔIC fragment were 5′-CAAGTATGCTGATCCAGATCGGGA (nt 1827–1850 from ErbB4 open reading frame; Plowman et al. 1993) and 5′-GAATTCTTATTACTTGTCATCGTC, which hybridizes to the 3′-terminal Flag epitope tag and unique EcoRI site of MMTV-ErbB4ΔIC.
RNA Isolation and RNase Protection Assay
RNA was isolated from the number 4 inguinal mammary gland by TRIzol extraction (GIBCO BRL). Riboprobe synthesis and purification, and RNA analysis using the RPA II ribonuclease protection assay kit (Ambion) were performed as described (Jones and Stern 1999).
Tissue Preparation for Histological Analysis
For hematoxylin/eosin staining, immunohistochemistry, and in situ hybridization, a portion of the number four inguinal mammary gland was spread onto a glass microscope slide and fixed in freshly prepared 4% paraformaldehyde in PBS (15 mM Na2HPO4, 1.5 mM KH2PO4, 137 mM NaCl, 3 mM KCl, pH 7.2) overnight at 4°C. The fixed tissue was embedded in paraffin and 6-μm sections were dried onto gelatin-coated slides using standard procedures.
Immunohistochemistry
Immunohistochemical detection of Flag-tagged ErbB4ΔIC and Stat5 was performed as described elsewhere (Jones and Stern 1999) with the following modifications. For Flag immunohistochemistry, the primary antibody was rabbit anti-Flag probe (Santa Cruz) diluted to 0.67 μg/ml and secondary antibody was biotinylated goat anti–rabbit diluted to 15 μg/ml (Vector Labs, Inc.). Negative controls included similarly treated mammary gland paraffin sections from nontransgenic siblings and affinity-purified rabbit IgG diluted to 0.67 μg/ml as the primary antibody. For Stat5 immunohistochemistry, primary antibody was rabbit anti-Stat5 LH743 (Liu et al. 1996a; generously provided by Dr. Lothar Hennighausen, National Institutes of Health, Bethesda, MD) diluted 1/600 and secondary antibody was biotinylated goat anti–rabbit diluted to 15 μg/ml (Vector Labs, Inc.). Sections were blocked and all antibodies were diluted in PBS containing 10% goat serum. Similarly treated mammary gland paraffin sections from nontransgenic mice at 3- and 18-d postpartum served as Stat5 positive and negative controls, respectively. An additional Stat5 negative control was rabbit serum (Pierce Chemical Co.) diluted 1/600 as the primary antibody.
For immunohistochemical detection of Stat5 phosphorylated at Y694, sections were pretreated to expose phosphorylated Stat5 epitopes. Deparaffinized sections were treated with 1 mg/ml of buffered trypsin (Sigma Chemical Co.) for 20 min at 37°C. Endogenous peroxidase activity was inactivated by incubating the sections in 0.5% H2O2 in PBS for 15 min at room temperature. The sections were incubated in 2 N HCl for 1 h at room temperature followed by two washes in 100 mM borate buffer, pH 8.5, for 5 min per wash. The sections were treated with 0.2% NP-40 for 30 min at room temperature. Between each treatment the sections were washed twice in PBS for 5 min per wash. The remainder of the procedure was performed as described elsewhere (Jones and Stern 1999) with the following modifications. Primary antibody was rabbit antiphospho-Stat5 (Zymed Labs, Inc.) diluted to 10 μg/ml and secondary antibody was biotinylated goat anti–rabbit diluted to 15 μg/ml (Vector Labs, Inc.). PBS containing 10% goat serum was used to block sections and dilute antibodies. For peptide blocking experiments, phospho-Stat5 antibody was diluted to 10 μg/ml in PBS containing 10% goat serum and 400 μg/ml of phospho-Stat5 peptide immunogen (Zymed Labs, Inc.). The antibody/peptide solution was incubated for 15 min at room temperature before application to the sections. Positive and negative controls for phospho-Stat5 immunohistochemistry were similarly treated paraffin sections from mammary glands at 3- and 18-d postpartum, respectively. Affinity-purified rabbit IgG diluted to 10 μg/ml as the primary antibody also served as a negative control.
Immunostained sections were lightly counterstained in hematoxylin (Polysciences Inc.) or methyl green (Vector Labs, Inc.) according to the manufacturer's instructions, dehydrated in EtOH, cleared in xylene, and coverslipped with Permount (Fisher Scientific Co.).
Riboprobe Synthesis and Purification
Buffers used for riboprobe synthesis and transcript purification were generally pretreated with DEPC. For in situ hybridization experiments, DNA template was linearized for sense and antisense riboprobe synthesis and contaminating ribonucleases were inactivated by proteinase treatment at 37°C for 1 h in 10 mM Tris, pH 8.0, 50 mM NaCl, 5 mM EDTA, 0.6% SDS, and 150 μg/ml proteinase K. In vitro transcription and subsequent DNase treatment were performed using a MAXIscript in vitro transcription kit (Ambion) with 1 μg of template DNA and 130 μCi of 35S-UTP (DuPont-NEN) exactly as described by the manufacturer. Transcripts were suspended to 200 μl in a final concentration of 10 mM DTT (Sigma Chemical Co.), 300 mM NaOAc, and 20 μg of t-RNA, and purified by EtOH precipitation with 2 M NH4OAc. The precipitated RNA was washed extensively in 70% EtOH, resuspended into 10 mM DTT, and precipitated and washed a second time. The final RNA pellet was resuspended into 10 mM DTT.
In Situ Hybridization
In situ hybridization was performed on 6-μm paraffin sections of mammary glands from female mice at 1- and 12-d postpartum using 35S-UTP labeled riboprobes. Sections were deparaffinized in xylene, washed in 100% EtOH, and defatted by incubating with chloroform for 5 min. The sections were hydrated through a descending EtOH series and washed in PBS for 5 min. The tissue was etched in 2 μg/ml of protease K in PBS for 10 min at 37°C and rinsed in PBS. Tissue sections were postfixed in 4% paraformaldehyde in PBS for 10 min, quenched with 0.2% glycine in PBS for 5 min, and washed in PBS for 5 min. Nonspecific binding sites were blocked by incubating the sections for 10 min in 100 mM triethanolamine (Sigma Chemical Co.), pH 8.0, 0.9% NaCl, containing 0.25% acetic anhydride (Sigma Chemical Co.). The slides were washed in 2× SSC (20× SSC = 3 M NaCl, 0.3 M sodium citrate, pH 7.0) for 5 min, dehydrated through an ascending ethanol series, treated with chloroform for 5 min, washed two times with 100% ethanol for 2 min per wash, and air dried.
The hybridization mixture contained 10 mM Tris, pH 7.5, 600 mM NaCl, 2 mM EDTA, 10 mM DTT, 1× Denhardt's (Sigma Chemical Co.), 500 μg/ml total yeast RNA (Ambion), 100 μg/ml poly-A (Pharmacia Biotech, Inc.), 100 mg/ml dextran sulfate (Sigma Chemical Co.), 50% deionized formamide (Ambion), and 4 × 104 cpm/μl of 35S-UTP–labeled riboprobe, and was heated at 80°C for 10 min immediately before use. To each section, 50 μl of hybridization mixture was applied, the sections were overlaid with parafilm coverslips, and hybridized at 50°C for 16 h in a humid chamber containing 10 mM Tris, pH 7.5, 600 mM NaCl, 2 mM EDTA, and 50% formamide (Sigma Chemical Co.). After hybridization, the parafilm coverslips were removed and the slides were washed twice at low stringency for 15 min per wash at 50°C in 2× SSC, 50% formamide, and 0.1% β-mercaptoethanol. Nonhybridized probe was digested by placing the slides in 10 mM Tris, pH 8.0, 500 mM NaCl, containing 20 μg/ml of RNase A (Sigma Chemical Co.) for 30 min at 37°C. The low stringency washes were repeated and the slides were washed an additional two times in 0.1× SSC and 1% β-mercaptoethanol at 50°C for 15 min per wash. The slides were dehydrated through an ascending ethanol series, with a final concentration of 600 mM NaCl included in ethanol solutions under 80%, and air dried. Dried slides were dipped in Kodak NTB-2 nuclear track emulsion diluted 1:1 with ddH2O at 45°C, and were exposed at 4°C in light-tight slide boxes containing silica gel desiccant packets (Sigma Chemical Co.). Before developing, the slides were warmed to room temperature and developed in Kodak D-19 developer for 2.5 min, washed in ddH2O for 30 s, fixed in Kodak fixer for 3 min, and washed in running tap water for 15 min. The sections were lightly counterstained with hematoxylin using the same procedure described for immunohistochemistry.
Cell Transfections
293T cells were transfected using FuGENE6 transfection reagent (Boehringer Mannheim Corp.) according to the manufacturer's instructions. In brief, cells 25% confluent in 100-mm tissue culture dishes were transfected with 500 μl of growth medium without serum, containing 10 μl of FuGENE6 and 2 μg of each plasmid, for a total of 4 μg. The cells were incubated with transfection mixture for 48 h in a humidified incubator at 37°C with 5% CO2. Plasmid pLXSN (Miller and Rosman 1989) served as a vector control for ErbB4 transfections. pLXSN-ErbB4 expresses human ErbB4 (Riese et al. 1995). pEF-neo served as a vector control for Stat5a transfections. pEF-Stat5a (Welte et al. 1999) encodes mouse Stat5a cDNA. pEF-Stat5/Y694F (Welte et al. 1999) is pEF-Stat5a with a tyrosine to phenylalanine mutation at residue 694, which abrogates Stat5 nuclear localization and DNA binding (Gouilleux et al. 1994). pEF-Stat5/R618V is pEF-Stat5a with an arginine to valine mutation at residue 618, which ablates SH2 function (Schindler and Darnell 1995). This mutation was created using QuikChange Site-Directed Mutagenesis Kit (Stratagene) and the oligonucleotide primer 5′-GCGAAAGCAGTCGACGGATTCGTGAAGCCACAG.
Immunoprecipitation from Cell Extracts and Western Blot Analysis
Transfected 293T cells were lysed in 2.0 ml of ice-cold EBC buffer (50 mM Tris, pH 7.5, 120 mM NaCl, 0.5% NP-40, with 1× Complete protease inhibitor cocktail [Boehringer Mannheim Corp.], 1 mM phenylmethylsulfonyl fluoride, and 1 mM pervanadate) on ice for 10 min. The cell lysates were cleared by centrifugation in a SS-34 rotor at 5,000 rpm for 15 min at 4°C. Immunoprecipitation of ErbB4 and Stat5a from 500 μl of lysate was performed by adding 50 μl of preswollen protein A–Sepharose (Pharmacia Biotech, Inc.) and 2 μg of anti-ErbB4 (C-18; Santa Cruz) or 2 μg of anti-Stat5b (C-17; Santa Cruz; Stern et al. 1986). Precipitated proteins were resolved by SDS-PAGE on a 7.5% acrylamide gel and the resolving gel was transferred to Trans-Blot 0.45-μm nitrocellulose (Bio-Rad Laboratories) using standard procedures. Western blot analysis was performed as described previously (Stern et al. 1986). For detection of phosphotyrosine containing proteins, mAb 4G10 (Upstate Biotechnology Inc.) was diluted to 1 μg/ml, followed by sheep anti–mouse conjugated with HRP (Nycomed Amersham Inc.) diluted 1:3,000. For detection of Stat5a phosphorylated at Y694, rabbit antiphospho-Stat5 (Zymed Labs, Inc.) was diluted to 0.5 μg/ml; for detection of ErbB4, rabbit polyclonal ErbB4 (C-18) was diluted to 0.2 μg/ml; and for detection of Stat5, rabbit polyclonal Stat5b (C-17) was diluted to 0.2 μg/ml. The secondary antibody for rabbit primary antibodies was donkey anti–rabbit conjugated with HRP (Nycomed Amersham Inc.) diluted 1:3,000. Signal was detected using SuperSignal West Pico chemiluminescence substrate (Pierce Chemical Co.) according to the manufacturer's instructions.
Results
Transgenic Mice Derived from MMTV-ErbB4ΔIC
Since the embryonic lethality of ErbB4 gene disruption precludes characterization of postnatal mammary development (Gassmann et al. 1995), we instead used a dominant-negative strategy to inactivate ErbB4 in the mammary gland. For this purpose, ErbB4 coding sequences encoding a receptor lacking the cytoplasmic domain were placed under the regulatory control of the MMTV promoter. pMMTV-ErbB4ΔIC encodes a protein of ∼120 kD, as determined by anti-Flag Western blot analysis of stably transfected FR3T3 cells (data not shown). Expression of pMMTV-ErbB4ΔIC inhibits EGF-stimulated tyrosine phosphorylation of the endogenous EGFR, verifying that, like cytoplasmic deletion mutants of other receptor tyrosine kinases, ErbB4ΔIC has dominant-negative activity (data not shown). The in vivo specificity of pMMTV-ErbB4ΔIC dominant-negative activity is described in the Discussion.
To determine the effect of dominant-negative ErbB4 activity within the developing mammary gland, transgenic mice were derived by injecting MMTV-ErbB4ΔIC DNA into the pronuclei of fertilized one-cell zygotes from B6SJL/F2 mice. 19 founders with transgene integration (identified by PCR) were crossed into an FVB strain, and transgene expression by the F2 female offspring was determined by RNase protection analysis. Transgene expression was detected in mammary glands of five week-old female offspring from six different founders (data not shown). The highest levels of transgene expression were observed in the offspring of founders 5963 and 5997. Phenotypic analysis of mammary glands from mice expressing ErbB4ΔIC was performed on F3 females derived by crossing founder line 5963 F2 mice with FVB strain mice. The phenotype of line 5963 was confirmed by analysis of the second founder line, 5997.
Expression of the MMTV-ErbB4ΔIC Transgene in the Mammary Gland
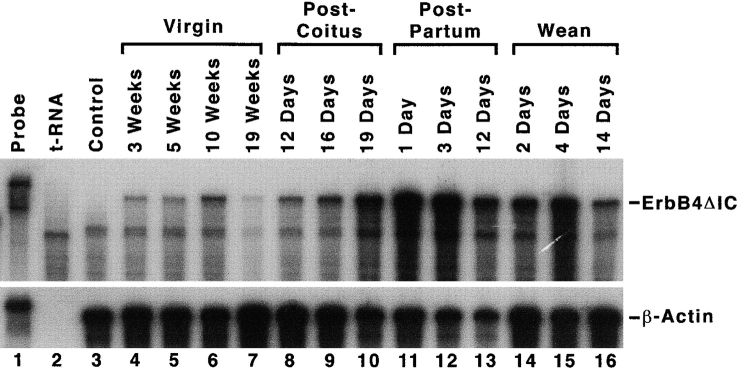
The temporal expression pattern of ErbB4ΔIC RNA in the mammary gland was determined by RNase protection assay (Fig. 1). The riboprobe hybridizes to the extreme 3′ end of the ErbB4ΔIC transgene, including unique sequences encoding the tandem Flag epitope tags, resulting in a protected fragment of 285 bp (Fig. 1, lanes 4–16). Transgene expression was first detected in prepubescent females at 3 wk and expression levels increased slightly with age, reaching maximal expression in the mature nulliparous mammary gland at 10 wk (Fig. 1, lanes 4–6). The apparent decrease in expression at 19 wk (Fig. 1, lane 7) was not observed in other experiments. Expression levels were similar from early to mid-pregnancy (Fig. 1, lanes 8 and 9), increased at late pregnancy (lane 10), were highest at 1- and 3-d postpartum (Fig. 1, lanes 11 and 12), and were reduced from 12-d postpartum (Fig. 1, lane 13) through weaning (Fig. 1, lanes 14–16).
Figure 1.
Expression analysis of ErbB4ΔIC RNA in developmentally staged mammary glands. RNA (20 μg) isolated from the number 4 inguinal mammary gland was hybridized with 32P-labeled antisense riboprobe corresponding to the COOH-terminal 285-bp of ErbB4ΔIC, including sequences encoding the tandem Flag epitope tags, and subjected to RNase protection analysis (upper panel). Negative controls included 20 μg each of tRNA and RNA from a nontransgenic sibling at 1-d postpartum (control). A probe that hybridizes to β-actin sequences was included to control for RNA integrity and to confirm that equivalent amounts of RNA were added to each reaction (lower panel). Protected fragments corresponding to ErbB4ΔIC and β-actin are indicated.
ErbB4ΔIC Protein Expression Is Associated with Condensed Lobuloalveoli during Lactation
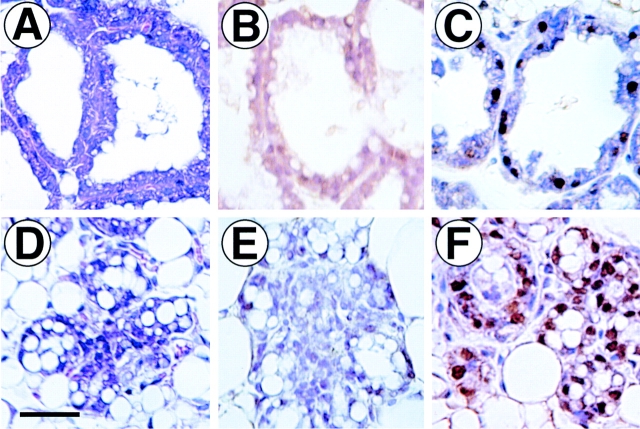
To determine the effects of ErbB4ΔIC expression on female mammary gland development, wholemounts and histological sections were examined from virgin mice at 3, 5, 6, 8, 10, and 19 wk of age; during early (12 d), mid- (16 d), and late (19 d) pregnancy; after parturition at days 3, 6, 9, 12, 15, or 18; and 2–4 d after weaning. At least three mice were analyzed at each time point. Despite the extensive time frame for transgene expression, and the fact that expression was highest shortly after parturition (Fig. 1), the only identifiable phenotypes were detected on day 12 postpartum. The fat pad of a nontransgenic mouse at 12-d postpartum is completely invested with engorged lobuloalveoli displacing stromal adipose cells. Secretory activity is demonstrated by lumens lined with protruding secretory epithelium (Fig. 2 A, arrow). Engorged active secretory lobuloalveoli were also observed in ErbB4ΔIC-expressing mice at 12-d postpartum (Fig. 2 B, arrow). In some transgenic mice (3 out of 5 examined), however, a subpopulation of lobuloalveoli failed to expand and contained an unusually high level of lumenal secretory lipids (Fig. 2 B, asterisk). Adipose cells were still abundant in this region of the mammary gland fat pad. The condensed lobuloalveoli resembled undifferentiated lobuloalveoli that are normally predominant during late pregnancy. We next used anti-Flag immunohistochemistry to determine if the condensed lobuloalveoli expressed the Flag-tagged ErbB4ΔIC transgene. Intense cytoplasmic immunostaining of epithelium within condensed lobuloalveoli was observed (Fig. 2 D, asterisks). Anti-Flag immunostaining was not observed in distended lobuloalveoli in the same tissue sections (Fig. 2 D, arrow). The lack of detectable transgene expression in this subpopulation of lobuloalveoli may be a result of variegated transgene expression. Variegated promoter expression within the mouse mammary gland has been reported for several mammary specific promoters, including the MMTV LTR promoter used in this study (Faerman et al. 1995; Deckard-Janatpour et al. 1997; Jones and Stern 1999).
Figure 2.
Immunohistochemical detection of ErbB4ΔIC protein in the mammary gland at 12-d postpartum. Paraffin-embedded section from a 12-d-postpartum nontransgenic sibling control stained with hematoxylin/eosin (A). Sequential sections (B–D) from a 12-d-postpartum mammary gland from an ErbB4ΔIC-expressing mouse were stained with hematoxylin/eosin (B), or stained by immunohistochemistry with rabbit IgG control antibody (C) or a rabbit anti-Flag antibody (D). Expanded secretory lobuloalveoli are indicated by arrows, and condensed atypical lobuloalveoli are indicated by asterisks. Bar in C, 100 μm.
Although the alveolar condensation associated with high ErbB4ΔIC expression might be caused by selective growth inhibition or apoptosis, neither BrdU incorporation experiments, nor TUNEL analysis revealed differences between the phenotypically normal and condensed lobuloalveolar populations in ErbB4ΔIC animals (data not shown). These results suggest instead that ErbB4ΔIC expression inhibits normal lobuloalveolar development and function at 12-d postpartum.
ErbB4ΔIC Expression Inhibits Milk Gene Expression
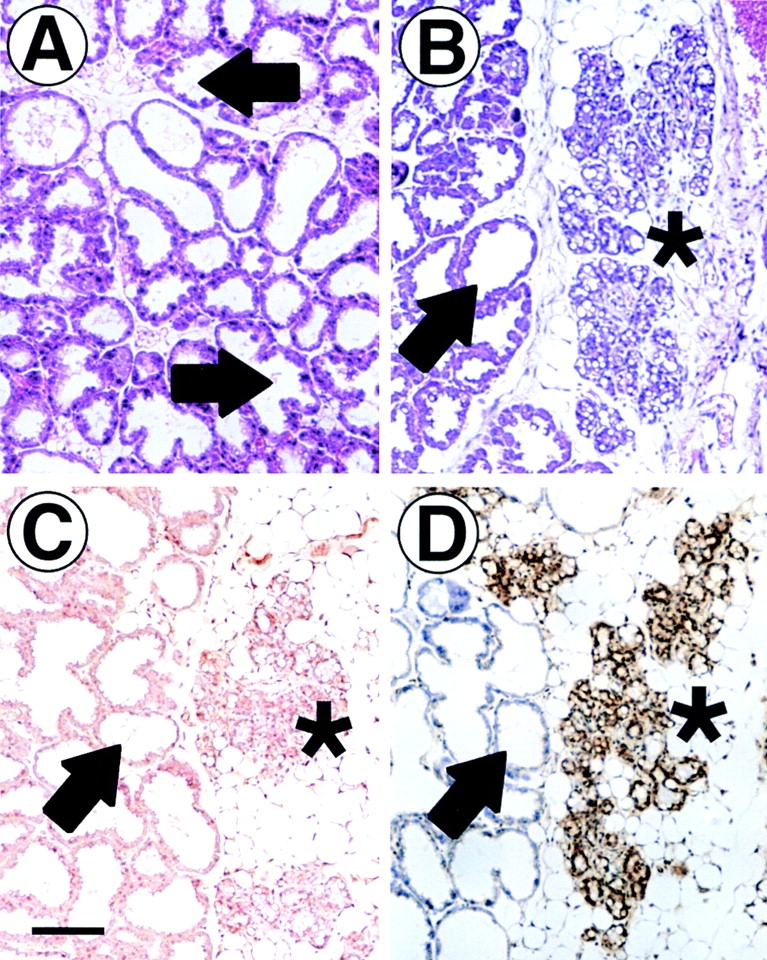
ErbB4ΔIC expression at 12-d postpartum impaired lobuloalveolar development, resulting in condensed alveolar structures with pronounced lipid secretory activity. These structures resembled normal undifferentiated lobuloalveoli observed at late pregnancy and parturition. To determine if the ErbB4ΔIC-expressing lobuloalveoli were lactationally active, we performed in situ hybridization using antisense riboprobes specific for the milk genes β-casein, WAP, and α-lactalbumin. Serial paraffin sections containing both normal expanded lobuloalveolar structures and condensed lobuloalveoli were examined (Fig. 3 A, arrow and asterisks, respectively). ErbB4ΔIC expression within condensed lobuloalveoli was confirmed by anti-Flag immunohistochemistry (Fig. 3 B, asterisks). The sense probes for β-casein, WAP, and α-lactalbumin yielded similar levels of background hybridization in both expanded and condensed lobuloalveoli (Fig. 3C, Fig. E, and Fig. G, arrows and asterisks, respectively). With antisense probe, equivalent high levels of β-casein RNA expression was observed in both the normal and ErbB4ΔIC-expressing lobuloalveoli (Fig. 3 D, arrow and asterisks, respectively). However, the ErbB4ΔIC-expressing lobuloalveoli showed a moderate diminution in WAP expression (Fig. 3 F). Strikingly, α-lactalbumin expression was reduced to sense probe background levels in condensed areas, but not in normal areas of the same section (Fig. 3 H). The decrease in WAP and the absence of α-lactalbumin expression suggests that terminal differentiation in ErbB4ΔIC-expressing lobuloalveolar epithelium has been disrupted. Similar in situ hybridization analysis performed on mammary glands from female mice at 1-d postpartum yielded equivalent levels of expression of these genes in transgenic and nontransgenic sisters (data not shown).
Figure 3.
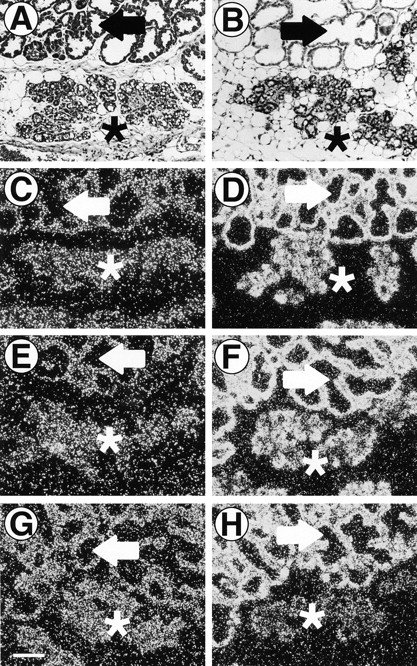
In situ hybridization analysis of milk protein gene expression in ErbB4ΔIC-expressing mammary glands at 12-d postpartum. Sequential sections from a 12-d-postpartum mammary gland from an ErbB4ΔIC-expressing mouse were stained with hematoxylin/eosin (A) or stained by immunohistochemistry with anti-Flag antibody (B). Additional sequential sections were analyzed by in situ hybridization with β-casein sense (C) or antisense (D) riboprobes, WAP sense (E) or antisense (F) riboprobes, or α-lactalbumin sense (G) or antisense (H) riboprobes. Expanded secretory lobuloalveoli are indicated by arrows and condensed atypical lobuloalveoli are indicated by asterisks. Bar in G, 100 μm.
Stat5 Localized to the Nucleus of ErbB4ΔIC-expressing Mammary Epithelium Is Not Phosphorylated at Y694
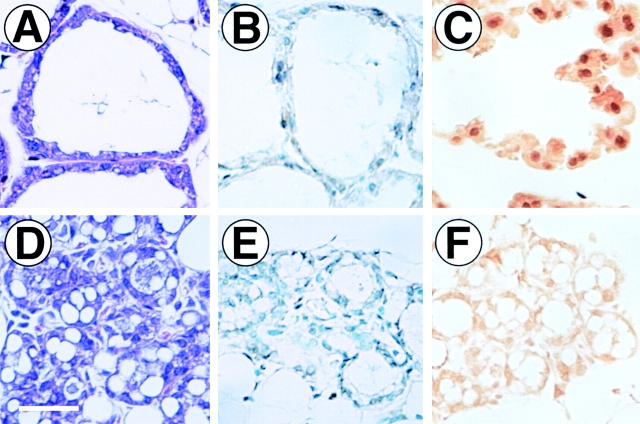
The condensed lobuloalveoli and pattern of impaired milk gene expression observed in ErbB4ΔIC-expressing mammary tissue resembles mammary defects observed in mice with Stat5 gene disruptions (Liu et al. 1996b; Teglund et al. 1998). Stat5 expression was determined by immunohistochemistry in sections of mammary glands at 12-d postpartum, containing both normal (Fig. 4 A) and ErbB4ΔIC-expressing lobuloalveoli (Fig. 4 D). Strong immunostaining was detected in the nuclei of both normal (compare Fig. 4B and Fig. C) and ErbB4ΔIC-expressing lobuloalveoli (compare Fig. 4E and Fig. F).
Figure 4.
Immunohistochem- ical detection of Stat5 protein in ErbB4ΔIC-expressing mammary glands at 12-d postpartum. High magnification photomicrographs of expanded lobuloalveoli lacking detectable ErbB4ΔIC expression (A–C) or a different region of the same section containing condensed lobuloalveoli expressing high levels of ErbB4ΔIC protein (D–F). Sections were stained with hematoxylin/eosin (A and D), or stained by immunohistochemistry with rabbit serum negative control (B and E) or rabbit anti-Stat5 (C and F). Bar in D, 30 μm.
Since functional Stat5 is phosphorylated at Y694 (reviewed in Groner and Gouilleux 1995), we used an antibody specific for Stat5 phosphorylated at Y694 to evaluate the phosphorylation state of Stat5 (Fig. 5). Strong nuclear staining and moderate cytoplasmic staining of phosphorylated Stat5 was detected within normal lobuloalveolar epithelium at 12-d postpartum (Fig. 5 C). Immunoreactivity was blocked by preadsorption with the peptide immunogen (Fig. 5 B) and was undetectable in sections incubated with affinity-purified rabbit IgG control primary antibody (data not shown). Immunoreactive Stat5 and Phospho-Stat5 were detected in both normal mammary glands and phenotypically normal areas of transgenic mammary glands at 1-, 3-, 6-, 9-, and 15-d postpartum, but not at day 18 (data not shown). However, at day 12 postpartum, Stat5 was localized to the nucleus, but not phosphorylated in areas expressing ErbB4ΔIC (Fig. 5 F). The lack of Y694 phosphorylation of nuclear Stat5 in ErbB4ΔIC-expressing lobuloalveolar epithelium suggests that it is functionally inactive.
Figure 5.
Immunohistochem- ical detection of Stat5 phosphorylated at Y694 in ErbB4ΔIC-expressing mammary glands at 12-d postpartum. High magnification representations of expanded lobuloalveoli lacking detectable ErbB4ΔIC expression (A–C) or a different region of the same section containing condensed lobuloalveoli expressing high levels of ErbB4ΔIC protein (D–F). Sections were stained with hematoxylin/eosin (A and D), or stained by immunohistochemistry with antiphospho-Stat5 antibody preadsorbed with peptide immunogen and counterstained with methyl green (B and E), or phospho-Stat5 antibody without counterstain (C and F). Bar in D, 30 μm.
ErbB4 and Stat5 Interaction and ErbB4 Mediated Phosphorylation of Stat5 at Y694 Requires a Functional Stat5 SH2 Domain
Since expression of ErbB4ΔIC appears to inhibit phosphorylation of Stat5 at the regulatory site Y694, it is possible that ErbB4 normally regulates this effector protein during mammary development. To determine if ErbB4 can induce phosphorylation of Stat5a at this site, the proteins were ectopically expressed at high levels in human embryonic kidney 293T cells (Fig. 6). Despite high levels of Stat5a expression in transfected cell lysates (Fig. 6 H, lanes 9 and 10, open circle), significant Stat5a tyrosine phosphorylation was observed only when Stat5a was coexpressed with ErbB4 (Fig. 6 E, lane 10, open circle). This phosphorylation included Y694, since it was detected by the anti-Stat5 phospho-Y694 antibody (Fig. 6 G, lane 10). When Stat5a and ErbB4 were coexpressed in 293T cells, they could be cross-coimmunoprecipitated (Fig. 6 D, lane 4, E and F, lane 10). Stat5a coimmunoprecipitated with anti-ErbB4 (Fig. 6 D, lane 4) and was not phosphorylated at Y694 (Fig. 6 C, lane 4), suggesting that phosphorylation of Stat5a results in rapid release of Stat5a from an ErbB4/Stat5a complex.
Figure 6.
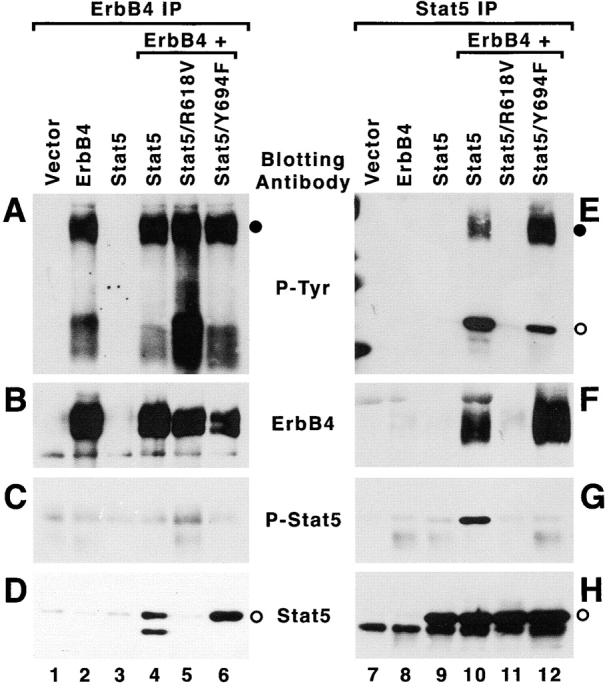
Coexpression of ErbB4 and Stat5a. 293T cells were transfected with various combinations of ErbB4 and Stat5 expression vectors and lysates of transfected cells were prepared 48 h posttransfection, as described in Materials and Methods. Immunoprecipitations were performed using ErbB4-specific (A–D) or Stat5-specific (E–H) antibodies. Immunoprecipitates were resolved by SDS-PAGE and the 7.5% acrylamide resolving gel was transferred to nitrocellulose. Western blot analysis was performed using antibodies to detect tyrosine phosphorylated proteins (A and E), ErbB4 (B and F), phospho-Stat5 (C and G), or Stat5 (D and H). Stat5 mutants were Stat5/R618V, a point mutation which ablates SH2 function, and Stat5/Y694F, which eliminates the regulatory Y694. Transfections were with empty vectors (lanes 1 and 7), ErbB4 alone (lanes 2 and 8), Stat5a alone (lanes 3 and 9), ErbB4 + Stat5a (lanes 4 and 10), ErbB4 + Stat5/R618V (lanes 5 and 11), and ErbB4 + Stat5/Y694F (lanes 6 and 12). Closed circles in A and E indicate the position of ErbB4 at ∼190 kD; open circles in D, E, and H indicate the position of Stat5a at ∼95 kD.
To determine the specificity of ErbB4/Stat5a interaction and ErbB4-mediated phosphorylation of Stat5a on Y694, 293T cells were transfected with mutant STAT5a alleles, with inactivating mutations in the SH2 domain (R618 to V) or at Y694 (Y to F). The two Stat5a mutants were expressed at levels comparable to wild-type Stat5a (Fig. 6 H, compare lanes 9 and 10 to 11 and 12), but the Stat5a mutants were not phosphorylated at Y694 when coexpressed with ErbB4 (Fig. 6 G, lanes 11 and 12). Interestingly, the Stat5a Y694F mutant was tyrosine phosphorylated at sites other than Y694 when coexpressed with ErbB4 (Fig. 6 E, lane 12). Alternative tyrosine phosphorylation of the Stat5a Y694F mutant also has been observed in 293T cells when cotransfected with a T cell receptor and Lck tyrosine kinase (Welte, T., and X.-Y. Fu, unpublished observations), and with activation of the EGFR (Olayioye et al. 1999). When expressed alone, the Stat5a mutants Y694F and SH2 were not immunoprecipitated by ErbB4-specific antiserum (data not shown). In summary, ErbB4 and Stat5a were coimmunoprecipitated when coexpressed (Fig. 6 D, lane 6; E, closed circle, lane 12; and F, lane 12, respectively) and the Stat5a SH2 domain mutation prevented association of ErbB4 and Stat5a (Fig. 6 D, lane 5, E and F, lane 11). Hence, the interaction between activated ErbB4 and Stat5a, and subsequent tyrosine phosphorylation of Stat5a at Y694, requires a functional Stat5a SH2 domain.
Discussion
Members of the EGFR family have important functions during several stages of mammary gland development. Stromal expression of EGFR is required for ductal morphogenesis (Sebastian et al. 1998; Wiesen et al. 1999). Epithelial functions for the receptors are suggested by the patterns of expression of ligands and receptors (Schroeder and Lee 1998), by the functional effects of hormone implants (Vonderhaar 1987; Coleman et al. 1988; Jones et al. 1996; Kenney et al. 1996), and by the phenotypes of MMTV-driven transgenic animals expressing dominant-negative receptor genes (Xie et al. 1997; Jones and Stern 1999).
To elucidate the function of ErbB4 during mouse mammary gland development, we inactivated ErbB4 signaling in the developing mouse mammary gland through the directed expression of dominant-negative ErbB4 as a transgene. Despite significant levels of transgene expression throughout pregnancy and even greater levels of expression early postpartum, an ErbB4ΔIC-specific phenotype was not observed until mid-lactation at 12-d postpartum. Lobuloalveoli expressing ErbB4ΔIC at 12-d postpartum were condensed, with lumens predominantly filled with secretory lipids, a phenotype resembling normal tissue at late pregnancy. Furthermore, the ErbB4ΔIC-expressing lobuloalveoli failed to terminally differentiate, as evidenced by a lack of α-lactalbumin expression. ErbB4ΔIC also inhibited Stat5 phosphorylation at Y694, suggesting that Stat5 is an important downstream mediator of ErbB4 signaling during lactation.
The ErbB4ΔIC phenotype is significantly different from the phenotypes observed in transgenic mice harboring MMTV-driven dominant-negative EGFR or ErbB2 (Xie et al. 1997; Jones and Stern 1999). Dominant-negative EGFR inhibited ductal morphogenesis in the pubescent virgin mouse. The dominant-negative receptor did not have an effect, however, during pregnancy or lactation, apparently because of high levels of endogenous EGFR expression during these developmental stages. In contrast, the dominant-negative MMTV-ErbB2ΔIC did not affect virgin mammary gland development, but did inhibit lobuloalveolar development at parturition (Jones and Stern 1999). This phenotype appears earlier than the ErbB4ΔIC phenotype described here, and is not accompanied by suppression of mRNA for WAP, or α-lactalbumin (Jones, F., unpublished data). Although the dominant-negative receptors have some ability to inactivate heterologous dimerization partners in vitro, the nonoverlapping phenotypes obtained with dominant-negative EGFR, ErbB2, and ErbB4 suggests that each of these dominant-negative receptors does not act as a pan–dominant-negative.
Corroborative evidence supporting a role for ErbB4 signaling during mid-lactation comes from the timing of ErbB4 activation during mouse mammary gland development, since ErbB4 tyrosine phosphorylation is dramatically enhanced at 14-d postpartum (Schroeder and Lee 1998). These results support the conclusion that ErbB4 signaling plays an important role in lobuloalveolar maintenance and lactation during mid-lactation.
Additional members of the EGFR family and their ligands have been implicated in lobuloalveolar development and lactation. Our in vivo experiments identified a role for ErbB2 signaling in lobuloalveolar development at parturition (Jones and Stern 1999). Similarly, activated ErbB2 induces the formation of alveolar-like structures in a mammary epithelial cell culture system (Niemann et al. 1998). Waved-2 mice, which carry a spontaneous point mutation within the EGFR kinase domain, have reduced EGFR kinase activity and exhibit impaired lobuloalveolar development and decreased lactation (Fowler et al. 1995). The ErbB3 and ErbB4 ligand, NRG1, is required for lobuloalveolar development in mammary organ cultures (Yang et al. 1995) and can induce lobuloalveoli formation and lactation when encapsulated and implanted within mammary glands of virgin mice (Jones et al. 1996). In addition, mice lacking the EGFR ligand amphiregulin develop immature lobuloalveoli, and the cumulative loss of EGF and TGFα aggravates this defect (Luetteke et al. 1999). Overlapping expression and activation of ErbB4 with EGFR and ErbB2 during lobuloalveolar development (Schroeder and Lee 1998) suggests that ErbB4 activity at this developmental stage may be regulated by these receptors and the aforementioned ligands with functions during lobuloalveolar development.
The ErbB4ΔIC-expressing mammary epithelium resembles the phenotype observed in mice with a disrupted Stat5a gene (Liu et al. 1996b; Teglund et al. 1998). ErbB4 signaling during mid-lactation is required for Stat5 activation, since Stat5 expressed in ErbB4ΔIC-expressing lobuloalveoli was not phosphorylated on the regulatory Y694. Phosphorylation of this residue is essential for some Stat5 functions including dimerization and DNA binding (Groner and Gouilleux 1995). To our knowledge, this is the first in vivo evidence that an EGFR family member can mediate activation of Stat5. Surprisingly, Stat5 lacking phosphorylation at Y694 was localized to the nucleus of ErbB4ΔIC expressing lobuloalveolar epithelium. The current paradigm is that Stat5 phosphorylation at Y694 and subsequent dimerization are essential for Stat5 nuclear localization (Groner and Gouilleux 1995). However, this may not always be the case. It is conceivable that Stat is imported to the nucleus in a complex with the ligand-activated progesterone receptor (Richer et al. 1998). Johnson and coworkers have observed EGF-induced nuclear translocation of nonphosphorylated Stat2 (Johnson et al. 1999). These recent observations, along with our results, demonstrate that nuclear translocation of Stat proteins, mediated by activation of EGFR family members, can occur in the absence of Stat tyrosine phosphorylation at Y694. Others have detected ErbB4 within the nucleus of breast epithelium (Gullick and Srinivasan 1998), and nuclear translocation of exogenous ErbB4 ligand, NRG1, has been reported (Li et al. 1996). These results raise the possibility that Stat5 may be transported to the nucleus in a complex with ligand-bound receptor. A similar mechanism has been proposed for Stat nuclear translocation mediated by ligand-activated interferon γ receptor (Johnson et al. 1998).
The coupling of ErbB4 to Stat5 regulation is reinforced by a survey of the ability of ErbB family receptor combinations to regulate Stats (Olayioye et al. 1999). NRG1 stimulated Tyr phosphorylation and activation of Stat5b in fibroblasts coexpressing ErbB2 and ErbB4, but not fibroblasts coexpressing EGFR and ErbB4, or ErbB2 and ErbB3. NRG1 is expressed at moderate levels during lactation (Schroeder and Lee 1998), suggesting a means for activation of Stat5.
In transient transfection assays, ErbB4 induced phosphorylation of Stat5a on Y694, and the two proteins could be coprecipitated in a Stat5 SH2-dependent manner. This suggests that Stat5 is a direct substrate for ErbB4, although we cannot rule out the possible recruitment of a second tyrosine kinase into the complex. Indeed, c-src is an important mediator of Stat5a activation by ErbB family members, and Janus kinases (JAKs) can associate stably with these receptors (Olayioye et al. 1999). The COOH-terminal portion of ErbB4, which contains tyrosine autophosphorylation sites, is required for ErbB4 and Stat5a coimmunoprecipitation (data not shown). The Stat5 consensus docking site (YZXZ, where Z represents a hydrophobic residue; May et al. 1996), is present at three sites within the COOH terminus of ErbB4, raising the possibility of a direct interaction between Stat5 and ErbB4.
Activation of Stat5 during lactation is thought to be mediated by prolactin receptor (PrlR) signaling (reviewed in Hennighausen et al. 1997). In the rodent mammary gland, PrlR is expressed as long (LPrlR) and short (SPrlR) isoforms (Boutin et al. 1988; Davis and Linzer 1989; Shirota et al. 1990). Only the LPrlR can activate Stat5 to stimulate β-casein promoter activity (Lesueur et al. 1991). Moreover, SPrlR acts as a dominant-negative and interferes with β-casein promoter induction by LPrlR (Berlanga et al. 1997). Expression of the two PrlR isoforms is differentially regulated during mammary development, resulting in an increase in the ratio between SPrlR and LPrlR as lactation progresses (Jahn et al. 1991; Nagano and Kelly 1994; Mizoguchi et al. 1997). The formation of inactive SPrlR/LPrlR heterodimers would, therefore, increase during lactation. One possible function of ErbB4 signaling in the mouse mammary gland would be maintenance of Stat5 activation at mid-lactation and compensation for the potential diminution in PrlR signaling activity. Another possibility is based upon the mechanistic differences between EGFR and PrlR regulation of Stat5 (Olayioye et al. 1999). c-Src is required for coupling of EGFR, but not PrlR to Stat5, and, conversely, Jak2 is required for coupling of PrlR, but not EGFR to Stat5. Moreover, EGFR (Olayioye et al. 1999) and ErbB4 (see above) induce additional Tyr phosphorylation in Stat5 besides Y694. Hence, the downstream targets and adaptor functions of Stat5 may be different when regulated by receptor kinases or by cytokine receptors, so that Stat regulation by these two afferent systems is not functionally redundant.
In contrast to expression of the other EGFR family members, expression of ErbB4 in breast cancer is associated with favorable prognosis (Bacus et al. 1994, Bacus et al. 1996; Knowlden et al. 1998) and a differentiating tumor phenotype (Srinivasan et al. 1998). In this communication, we present in vivo evidence demonstrating a role for ErbB4 signaling in terminal differentiation of mammary epithelial cells. Our results raise the intriguing possibility that ErbB4 activity in breast cancer cells may activate differentiation pathways and thus antagonize the oncogenic properties of other coexpressed EGFR family members.
Acknowledgments
We are grateful to Carole Pelletier and David Brownstein at the Transgenic Mouse Shared Resource of the Yale University School of Medicine for producing transgenic animals. We thank Joe Jerry and John Wysolmerski for advice on in situ hybridization. We thank Lothar Hennighausen for supplying Stat5 antiserum. We thank Nancy Hynes for supplying mouse β-casein cDNA. We thank JoAnn Falato for exceptional administrative assistance. We thank Marc Schwartz, Dhara Amin, Jonathan McMenamin-Balano, and Amy Jackson-Fisher for critical reading of this manuscript. Finally, we thank Rajani Ramabhadran for excellent technical support and other members of the Stern lab for advice and critical insights. This work is dedicated to June Allison, a courageous survivor of breast cancer.
This work was supported by PHS grants R01CA45708 (to D.F. Stern and F. Jones), R01GM55590 (to X.-Y. Fu and T. Welte), the United States Army Medical Research and Material Command grant DAMD17-96-1-6158 (to F. Jones) and Austrian APART Fellowship (to T. Welte).
Footnotes
1.used in this paper: EGFR, epidermal growth factor receptor; LPrlR, long prolactin receptor isoform; MMTV, mouse mammary tumor virus; NRG1, neuregulin-1; PrlR, prolactin receptor; SPrlR, short prolactin receptor isoform; Stat, signal transducers and activators of transcription; TGFα, transforming growth factor α; WAP, whey acidic protein
Frank E. Jones' current address is University of Scranton, Institute of Molecular Biology and Medicine, Scranton, PA 18510.
References
- Alroy I., Yarden Y. The ErbB signaling network in embryogenesis and oncogenesissignal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83–86. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- Bacus S.S., Zelnick C.R., Plowman G., Yarden Y. Expression of the erbB-2 family of growth factor receptors and their ligands in breast cancersimplication for tumor biology and clinical behavior. Am. J. Clin. Pathol. 1994;102:S13–S24. [PubMed] [Google Scholar]
- Bacus S.S., Chin D., Yarden Y., Zelnick C.R., Stern D.F. Type 1 receptor tyrosine kinases are differentially phosphorylated in mammary carcinoma and differentially associated with steroid receptors. Am. J. Pathol. 1996;148:549–558. [PMC free article] [PubMed] [Google Scholar]
- Berlanga J.J., Garcia-Ruiz J.P., Perrot-Applanat M., Kelly P.A., Edery M. The short form of the prolactin (PRL) receptor silences PRL induction of the β-casein gene promoter. Mol. Endocrinol. 1997;11:1449–1457. doi: 10.1210/mend.11.10.9994. [DOI] [PubMed] [Google Scholar]
- Bobrow L.G., Millis R.R., Happerfield L.C., Gullick W.J. c-erbB-3 protein expression in ductal carcinoma in situ of the breast. Eur. J. Cancer. 1997;33:1846–1850. doi: 10.1016/s0959-8049(97)00244-x. [DOI] [PubMed] [Google Scholar]
- Boutin J.M., Jolicoeur C., Okamura H., Gagnon J., Edery M., Shirota M., Banville D., Dusanter-Fourt I., Djiane J., Kelly P.A. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor family. Cell. 1988;53:69–77. doi: 10.1016/0092-8674(88)90488-6. [DOI] [PubMed] [Google Scholar]
- Coleman S., Silberstein G.B., Daniel C.W. Ductal morphogenesis in the mouse mammary glandevidence supporting a role for epidermal growth factor. Dev. Biol. 1988;127:304–315. doi: 10.1016/0012-1606(88)90317-x. [DOI] [PubMed] [Google Scholar]
- David M., Wong L., Flavell R., Thompson S.A., Wells A., Larner A.C., Johnson G.R. STAT activation by epidermal growth factor (EGF) and amphiregulinrequirement for the EGF receptor kinase but not for tyrosine phosphorylation sites or JAK1. J. Biol. Chem. 1996;271:9185–9188. doi: 10.1074/jbc.271.16.9185. [DOI] [PubMed] [Google Scholar]
- Davis J.A., Linzer D.I.H. Expression of multiple forms of the prolactin receptor in mouse liver. Mol. Endocrinol. 1989;3:674–680. doi: 10.1210/mend-3-4-674. [DOI] [PubMed] [Google Scholar]
- Deckard-Janatpour K., Muller W.J., Chodosh L.A., Gardner H.P., Marquis S.T., Coffey R., Cardiff R.D. Differential expression of the neu transgene in murine mammary tissues. Int. J. Oncol. 1997;11:235–241. doi: 10.3892/ijo.11.2.235. [DOI] [PubMed] [Google Scholar]
- Dickson R.B., Lippman M.E. Growth factors in breast cancer. Endocrine Rev. 1995;16:559–589. doi: 10.1210/edrv-16-5-559. [DOI] [PubMed] [Google Scholar]
- Faerman A., Barash I., Puzis R., Nathan M., Hurwitz D.R., Shani M. Dramatic heterogeneity of transgene expression in the mammary gland of lactating micea model system to study the synthetic activity of mammary epithelial cells. J. Histochem. Cytochem. 1995;43:461–470. doi: 10.1177/43.5.7730585. [DOI] [PubMed] [Google Scholar]
- Fowler K.J., Walker F., Alexander W., Hibbs M.L., Nice E.C., Bohmer R.M., Mann G.B., Thumwood C., Maglitto R., Danks J.A. A mutation in the epidermal growth factor receptor in waved-2 mice has a profound effect on receptor biochemistry that results in impaired lactation. Proc. Natl. Acad. Sci. USA. 1995;92:1465–1469. doi: 10.1073/pnas.92.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R., Yu C.-L., Hudnall A., Catlett R., Nelson K.L., Smithgall T., Fujita D.J., Ethier S.P., Jove R. Constituitive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ. 1997;8:1267–1276. [PubMed] [Google Scholar]
- Gassmann M., Casagranda F., Orioli D., Simon H., Lai C., Klein R., Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Gouilleux F., Wakao H., Mundt M., Groner B. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J. 1994;13:4361–4369. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Gouilleux F. Prolactin-mediated gene activation in mammary epithelial cells. Curr. Opin. Genet. Dev. 1995;5:587–594. doi: 10.1016/0959-437x(95)80027-1. [DOI] [PubMed] [Google Scholar]
- Gullick W.J., Srinivasan R. The type 1 growth factor receptor familynew ligands and receptors and their role in breast cancer. Breast Cancer Res. Treat. 1998;52:43–53. doi: 10.1023/a:1006107016969. [DOI] [PubMed] [Google Scholar]
- Hennighausen L.G., Sippel A.E. Mouse whey acidic protein is a novel member of the family of “four-disulfide core” proteins. Nucleic Acids Res. 1982;10:2677–2684. doi: 10.1093/nar/10.8.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L., Robinson G.W., Wagner K.-U., Liu X. Prolactin signaling in mammary gland development. J. Biol. Chem. 1997;272:7567–7569. doi: 10.1074/jbc.272.12.7567. [DOI] [PubMed] [Google Scholar]
- Hynes N.E., Stern D.F. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim. Biophys. Acta. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Jahn G.A., Edery M., Belair L., Kelly P.A., Djiane J. Prolactin receptor gene expression in rat mammary gland and liver during pregnancy and lactation. Endocrinology. 1991;128:2976–2984. doi: 10.1210/endo-128-6-2976. [DOI] [PubMed] [Google Scholar]
- Johnson H.M., Torres B.A., Green M.M., Szente B.E., Siler K.I., Larkin J., III, Subramaniam P.S. Cytokine-receptor complexes as chaperones for nuclear translocation of signal transducers. Biochem. Biophys. Res. Commun. 1998;244:607–614. doi: 10.1006/bbrc.1998.8254. [DOI] [PubMed] [Google Scholar]
- Johnson L.R., McCormack S.A., Yang C.-H., Pfeffer S.R., Pfeffer L.M. EGF induces nuclear translocation of STAT2 without tyrosine phosphorylation in intestinal epithelial cells. Cell Physiol. 1999;45:C419–C425. doi: 10.1152/ajpcell.1999.276.2.C419. [DOI] [PubMed] [Google Scholar]
- Jones F.E., Stern D.F. Expression of dominant-negative ErbB2 in the mammary gland of transgenic mice reveals a role in lobuloalveolar development and lactation. Oncogene. 1999;18:3481–3490. doi: 10.1038/sj.onc.1202698. [DOI] [PubMed] [Google Scholar]
- Jones F.E., Jerry D.J., Guarino B.C., Andrews G.C., Stern D.F. Heregulin induces in vivo proliferation and differentiation of mammary epithelium into secretory lobuloalveoli. Cell Growth Differ. 1996;7:1031–1038. [PubMed] [Google Scholar]
- Kazansky A.V., Raught B., Lindsey S.M., Wang Y.-f. Regulation of mammary gland factor/Stat5 during mammary gland development. Mol. Endocrinol. 1995;9:1598–1609. doi: 10.1210/mend.9.11.8584036. [DOI] [PubMed] [Google Scholar]
- Kenney N.J., Smith G.H., Rosenberg K., Cutler M.L., Dickson R.B. Induction of ductal morphogenesis and lobular hyperplasia by amphiregulin in the mouse mammary gland. Cell Growth Differ. 1996;7:1769–1781. [PubMed] [Google Scholar]
- Knowlden J.M., Gee J.M.W., Seery L.T., Farrow L., Gullick W.J., Ellis I.O., Blamey R.W., Robertson J.F.R., Nicholson R.I. c-erbB3 and c-erbB4 expression is a feature of the endocrine responsive phenotype in clinical breast cancer. Oncogene. 1998;17:1949–1957. doi: 10.1038/sj.onc.1202107. [DOI] [PubMed] [Google Scholar]
- Lemoine N.R., Barnes D.M., Hollywood D.P., Hughes C.M., Smith P., Dublin E., Prigent S.A., Gullick W.J., Hurst H.C. Expression of the ERBB3 gene product in breast cancer. Br. J. Cancer. 1992;66:1116–1121. doi: 10.1038/bjc.1992.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesueur L., Edery M., Ali S., Paly J., Kelly P.A., Djiane J. Comparison of long and short forms of the prolactin receptor on prolactin-induced milk gene transcription. Proc. Natl. Acad. Sci. USA. 1991;88:824–828. doi: 10.1073/pnas.88.3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Park J.W., Nuijens A., Sliwkowski M.X., Keller G.-A. Heregulin is rapidly translocated to the nucleus and its transport is correlated with c-myc induction in breast cancer cells. Oncogene. 1996;12:2473–2477. [PubMed] [Google Scholar]
- Liu X., Robinson G.W., Hennighausen L. Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation Mol. Endocrinol. 10 1996. 1496 1506a [DOI] [PubMed] [Google Scholar]
- Liu X., Robinson G.W., Wagner K.-U., Garrett L., Wynshaw-Boris A., Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis Genes Dev. 11 1996. 179 186b [DOI] [PubMed] [Google Scholar]
- Luetteke N.C., Qui T.H., Fenton S.E., Troyer K.L., Riedel R.F., Chang A., Lee D.C. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126:2739–2750. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- May P., Gerhartz C., Heesel B., Welte T., Doppler W., Graeve L., Horn F., Heinrich P.C. Comparative study on the phosphotyrosine motifs of different cytokine receptors involved in STAT5 activation. FEBS Lett. 1996;394:221–226. doi: 10.1016/0014-5793(96)00955-6. [DOI] [PubMed] [Google Scholar]
- Miller A.D., Rosman G.J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989;7:980–990. [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi Y., Yamaguchi H., Aoki F., Enami J., Sakai S. Corticosterone is required for the prolactin receptor gene expression in the late pregnant mouse mammary gland. Mol. Cell. Endocrinol. 1997;132:177–183. doi: 10.1016/s0303-7207(97)00134-2. [DOI] [PubMed] [Google Scholar]
- Nagano M., Kelly P.A. Tissue distribution and regulation of rat prolactin receptor gene expressionquantitative analysis by polymerase chain reaction. J. Biol. Chem. 1994;269:13337–13345. [PubMed] [Google Scholar]
- Naidu R., Yadav M., Nair S., Kutty M.K. Expression of c-ErbB3 protein in primary breast carcinomas. Br. J. Cancer. 1998;78:1385–1390. doi: 10.1038/bjc.1998.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann C., Brinkmann V., Spitzer E., Hartmann G., Sachs M., Naundorf H., Birchmeier W. Reconstitution of mammary gland development in vitrorequirement of c-met and c-erbB2 signaling for branching and alveolar morphogenesis. J. Cell Biol. 1998;143:533–545. doi: 10.1083/jcb.143.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayioye M.A., Beuvink I., Horsch K., Daly J.M., Hynes N.E. ErbB receptor-induced activation of Stat transcription factors is mediated by Src tyrosine kinases. J. Biol. Chem. 1999;274:17209–17218. doi: 10.1074/jbc.274.24.17209. [DOI] [PubMed] [Google Scholar]
- Ornitz D.M., Moreadith R.W., Leder P. Binary system for regulating transgene expression in micetargeting int-2 gene expression with yeast GAL4/UAS control elements. Proc. Natl. Acad. Sci. USA. 1991;88:698–702. doi: 10.1073/pnas.88.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman G.D., Culouscou J.-M., Whitney G.S., Green J.M., Carlton G.W., Foy L., Neubauer M.G., Shoyab M. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc. Natl. Acad. Sci. USA. 1993;90:1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn C.M., Ostrowski J.L., Lane S.A., Loney D.P., Teasdale J., Benson E.A. c-erbB-3 protein expression in human breast cancer; comparison with other tumour variables and survival. Histopathology. 1994;25:247–252. doi: 10.1111/j.1365-2559.1994.tb01324.x. [DOI] [PubMed] [Google Scholar]
- Richer J.K., Lange C.A., Manning N.G., Owen G., Powell R., Horwitz K.B. Convergence of progesterone with growth factor and cytokine signaling in breast cancer. J. Biol. Chem. 1998;273:31317–31326. doi: 10.1074/jbc.273.47.31317. [DOI] [PubMed] [Google Scholar]
- Riese D.J., II, Stern D.F. Specificity within the EGF family/ErbB receptor family signaling network. BioEssays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Riese D.J., II, van Raaij T.M., Plowman G.D., Andrews G.C., Stern D.F. Cellular response to neuregulins is governed by complex interactions of the erbB receptor family. Mol. Cell. Biol. 1995;15:5770–5776. doi: 10.1128/mcb.15.10.5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff-Jamison S., Chen K., Cohen S. Epidermal growth factor induces the tyrosine phosphorylation and nuclear translocation of Stat 5 in mouse liver. Proc. Natl. Acad. Sci. USA. 1995;92:4215–4218. doi: 10.1073/pnas.92.10.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Darnell J.E. Transcriptional responses to polypeptide ligandsthe JAK-STAT pathway. Annu. Rev. Biochem. 1995;64:621–651. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- Schroeder J.A., Lee D.C. Dynamic expression and activation of ERBB receptors in the developing mouse mammary gland. Cell Growth Differ. 1998;9:451–464. [PubMed] [Google Scholar]
- Sebastian J., Richards R.G., Walker W.P., Wiesen J.F., Werb Z., Derynck R., Hom Y.K., Cunha G.R., DiAugustine R.P. Activation and function of the epidermal growth factor receptor and erbB-2 during mammary gland morphogenesis. Cell Growth Differ. 1998;9:777–785. [PubMed] [Google Scholar]
- Shirota M., Banville D., Ali S., Jolicoeur C., Boutin J.M., Edery M., Dijane J., Kelly P.A. Expression of two forms of prolactin receptor in rat ovary and liver. Mol. Endocrinol. 1990;4:1136–1143. doi: 10.1210/mend-4-8-1136. [DOI] [PubMed] [Google Scholar]
- Srinivasan R., Poulsom R., Hurst H.C., Gullick W.J. Expression of the c-erbB-4/HER4 protein and mRNA in normal human fetal and adult tissues and in a survey of nine solid tumour types. J. Pathol. 1998;185:236–245. doi: 10.1002/(SICI)1096-9896(199807)185:3<236::AID-PATH118>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Stern D.F., Heffernan P.A., Weinberg R.A. p185, a product of the neu proto-oncogene, is a receptor like protein associated with tyrosine kinase activity. Mol. Cell. Biol. 1986;6:1729–1740. doi: 10.1128/mcb.6.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teglund S., McKay C., Schuetz E., vanDeursen J.M., Stravopodis D., Wang D., Brown M., Bodner S., Grosveld G., Ihle J.N. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- Vilotte J.-L., Soulier S. Isolation and characterization of the mouse α-lactalbumin-encoding geneinterspecies comparison, tissue- and stage-specific expression. Gene. 1992;119:287–292. doi: 10.1016/0378-1119(92)90285-w. [DOI] [PubMed] [Google Scholar]
- Vonderhaar B.K. Local effects of EGF, α-TGF, and EGF-like growth factors on lobuloalveolar development of the mouse mammary gland in vivo. J. Cell. Physiol. 1987;132:581–584. doi: 10.1002/jcp.1041320324. [DOI] [PubMed] [Google Scholar]
- Wakao H., Schmitt N.M., Groner B. Mammary gland-specific nuclear factor is present in lactating rodent and bovine mammary tissue and composed of a single polypeptide of 89 kDa. J. Biol. Chem. 1992;267:16365–16370. [PubMed] [Google Scholar]
- Wakao H., Gouilleux F., Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte T., Leitenberg D., Dittel B.N., al-Ramadi B.K., Xie B., Chin Y.E., Janeway C.A., Bothwell A.L.M., Bottomly K., Fu X.-Y. STAT5 interaction with the T cell receptor complex and stimulation of T cell proliferation. Science. 1999;283:222–225. doi: 10.1126/science.283.5399.222. [DOI] [PubMed] [Google Scholar]
- Wiesen J.F., Young P., Werb Z., Cunha G.R. Signaling through the stromal epidermal growth factor receptor is necessary for mammary ductal development. Development. 1999;126:335–344. doi: 10.1242/dev.126.2.335. [DOI] [PubMed] [Google Scholar]
- Xie W., Paterson A.J., Chin E., Nabell L.M., Kudlow J.E. Targeted expression of a dominant negative epidermal growth factor receptor in the mammary gland of transgenic mice inhibits pubertal mammary duct development. Mol. Endocrinol. 1997;11:1766–1781. doi: 10.1210/mend.11.12.0019. [DOI] [PubMed] [Google Scholar]
- Yang Y., Spitzer E., Meyer D., Sachs M., Niemann C., Hartmann G., Weidner K.M., Birchmeier C., Birchmeier W. Sequential requirement of hepatocyte growth factor and neuregulin in the morphogenesis and differentiation of the mammary gland. J. Cell Biol. 1995;131:215–226. doi: 10.1083/jcb.131.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]