Abstract
Tight junctions (TJs) in endothelial cells are thought to determine vascular permeability. Recently, claudin-1 to -15 were identified as major components of TJ strands. Among these, claudin-5 (also called transmembrane protein deleted in velo-cardio-facial syndrome [TMVCF]) was expressed ubiquitously, even in organs lacking epithelial tissues, suggesting the possible involvement of this claudin species in endothelial TJs. We then obtained a claudin-6–specific polyclonal antibody and a polyclonal antibody that recognized both claudin-5/TMVCF and claudin-6. In the brain and lung, immunofluorescence microscopy with these polyclonal antibodies showed that claudin-5/TMVCF was exclusively concentrated at cell–cell borders of endothelial cells of all segments of blood vessels, but not at those of epithelial cells. Immunoreplica electron microscopy revealed that claudin-5/TMVCF was a component of TJ strands. In contrast, in the kidney, the claudin-5/TMVCF signal was restricted to endothelial cells of arteries, but was undetectable in those of veins and capillaries. In addition, in all other tissues we examined, claudin-5/TMVCF was specifically detected in endothelial cells of some segments of blood vessels, but not in epithelial cells. Furthermore, when claudin-5/TMVCF cDNA was introduced into mouse L fibroblasts, TJ strands were reconstituted that resembled those in endothelial cells in vivo, i.e., the extracellular face–associated TJs. These findings indicated that claudin-5/TMVCF is an endothelial cell–specific component of TJ strands.
Keywords: claudin, occludin, endothelium, tight junction, blood vessels
Endothelial cells provide a crucial interface between the blood and tissue environments. One of the important functions of endothelial cells is to determine and regulate vascular permeability, which is important not only in normal physiology to maintain the tissue environment but also in pathological conditions such as vasogenic edema and inflammation. Materials that are transported across the endothelial sheets do so via two distinct pathways: transcellular and paracellular pathways (for review see Spring 1998).
Tight junctions (TJs)1 occur not only in endothelial cells but also in simple epithelial cells. In these cells, TJs are thought to be directly involved in barrier and fence functions by sealing them to generate the primary barrier against the diffusion of solutes through the paracellular pathway and by acting as a boundary between the apical and basolateral plasma membrane domains to create and maintain cell polarity, respectively (for reviews see Schneeberger and Lynch 1992; Gumbiner 1987, Gumbiner 1993; Anderson and Van Itallie 1995; Yap et al. 1998). On ultrathin section electron micrographs, TJs appear as a set of discrete sites of apparent fusion involving the outer leaflet of plasma membranes of adjacent cells (Farquhar and Palade 1963). On freeze–fracture electron micrographs of most epithelial cells, TJs appear as a set of continuous, anastomosing intramembranous particle strands (TJ strands) in the protoplasmic face (P-face) with complementary grooves in the extracellular (E)-face (Staehelin 1973, Staehelin 1974).
The molecular architecture of TJs has been analyzed mainly using simple epithelial cells. Several peripheral membrane proteins such as ZO-1 (Stevenson et al. 1986), ZO-2 (Gumbiner et al. 1991), ZO-3 (Balda et al. 1993; Haskins et al. 1998), cingulin (Citi et al. 1988), 7H6 antigen (Zhong et al. 1993), and symplekin (Keon et al. 1996) were shown to be concentrated at the cytoplasmic surface of TJs. Occludin with four transmembrane domains was the first TJ-specific integral membrane protein (Furuse et al. 1993; Ando-Akatsuka et al. 1996). Occludin is thought to be not only a structural component (Fujimoto 1995; Furuse et al. 1996) but also a functional component of TJs; occludin was shown to be directly involved in barrier functions (McCarthy et al. 1996; Balda et al. 1996; Chen et al. 1997; Wong and Gumbiner 1997), in fence functions of TJs (Balda et al. 1996), and in cell adhesion (Van Itallie and Anderson 1997).
Recently, the occludin gene was disrupted in embryonic stem cells (Saitou et al. 1998). Unexpectedly, occludin-deficient visceral endoderm cells that were differentiated from occludin-knockout embryonic stem cells still bore a well-developed network of TJ strands, pointing to the existence of as yet unidentified TJ-specific integral membrane proteins. Recently, two novel integral membrane proteins, claudin-1 and -2, were identified, which were localized exclusively in TJ strands (Furuse et al. 1998a). Claudin-1 and -2 are structurally related (38% identical at the amino acid [aa] sequence level) and appear to bear four transmembrane domains, but show no sequence similarity to occludin. Furthermore, when these claudin cDNAs were singly introduced into L fibroblasts lacking TJs or claudin expression, a huge network of TJ strands was induced between stable transfectants (Furuse et al. 1998b). These findings indicated that claudins are major structural components of TJ strands (Tsukita and Furuse 1999).
A database similarity search identified many claudin-related sequences, and to date cDNAs encoding 13 more claudin-like proteins have been isolated, which were designated as claudin-3 to -15 (Morita et al. 1999a,Morita et al. 1999b; Tsukita and Furuse 1999). Northern blotting revealed that the tissue expression pattern varied significantly between different claudin species. Considering that endothelial cells express the specific cadherin isoform, vascular endothelial–cadherin (VE-cadherin) (Lampugnani et al. 1995), which shows unique characteristics, the question has naturally arisen as to whether there is an endothelial cell–specific claudin isoform. Claudin-5 (also called transmembrane protein deleted in velo-cardio-facial syndrome [TMVCF]) was suggested to be a good candidate for such an endothelial claudin, since it is expressed ubiquitously, even in organs lacking epithelial tissues such as the brain, skeletal muscle, and spleen (Morita et al. 1999a). In this study, we examined the expression and subcellular distribution of claudin-5/TMVCF by immunofluorescence microscopy as well as by immunoreplica electron microscopy, and found that this claudin species is an endothelial cell–specific component of TJ strands. We believe that this first description of the endothelial claudin will allow development of new methods with which to analyze the molecular mechanism of the regulation of vascular permeability.
Materials and Methods
Antibodies and Cells
Rat antioccludin mAb (MOC37) was described previously (Saitou et al. 1997). Anti–VE-cadherin mAb was a kind gift from Dr. N. Matsuyoshi (Kyoto University, Kyoto, Japan). Anti–platelet endothelial cell adhesion molecule (PECAM) polyclonal antibody (pAb) was purchased from PharMingen. Mouse L cells and their transfectants were cultured in DME supplemented with 10% FCS.
Production of pAbs
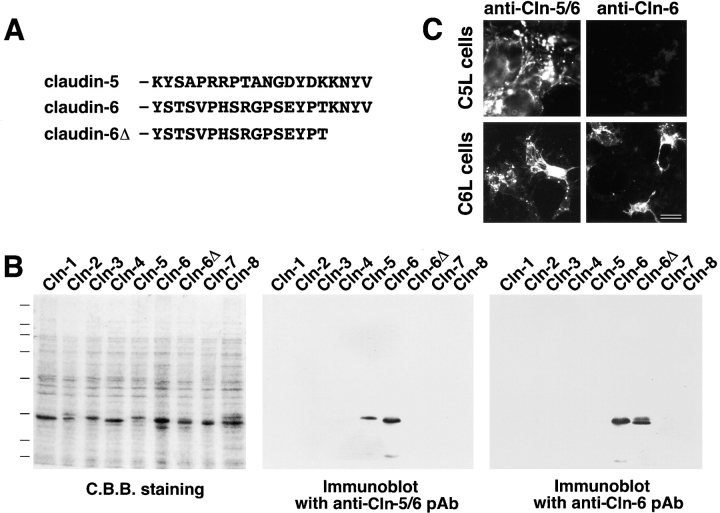
Two polypeptides, KYSAPRRPTANGDYDKKNYV and YSTSVPHSRGPSEYPTKNYV, which correspond to the COOH-terminal cytoplasmic domains of mouse claudin-5/TMVCF (aa 199–218) and claudin-6 (aa 200–219), respectively (a cysteine residue was added at their NH2 termini), were synthesized and coupled via the cysteine residue to keyhole limpet hemocyanin. These peptides were injected into rabbits as antigens. Rabbit antisera against the former and latter peptides were affinity-purified on nitrocellulose membranes with glutathione S-transferase (GST) fusion proteins with claudin-5/TMVCF and claudin-6Δ to obtain anti–claudin-5/6 pAb and anti–claudin-6 pAb, respectively (see Fig. 1 A).
Figure 1.
Specificity of pAbs. (A) Amino acid sequences of the cytoplasmic domains of claudin-5/TMVCF and claudin-6. The COOH-terminal KNYV is shared by these two claudin species. Two polypeptides corresponding to these sequences were used as antigens to produce pAbs in rabbits. (B) GST fusion proteins with the cytoplasmic domains of claudin-1 to -8 as well as the cytoplasmic domain of claudin-6 lacking its COOH-terminal KNYV (see claudin-6Δ in A) were produced in E. coli. The lysates of E. coli were separated by SDS-PAGE (C.B.B. staining), followed by immunoblotting with two distinct pAbs, which were raised against the cytoplasmic domain of claudin-5/TMVCF or claudin-6, respectively. The former recognized both GST–claudin-5/TMVCF and GST–claudin-6, but not GST–claudin-6Δ, indicating that this pAb (referred to as anti–claudin-5/6 pAb) specifically bound to the COOH-terminal KNYV sequence. The latter recognized GST–claudin-6 and GST–claudin-6Δ but not GST–claudin-5/TMVCF, indicating that this pAb (referred to as anti–claudin-6 pAb) was specific for the YSTSVPHSRGPSEYPT sequence of claudin-6 (see A). (C) Anti–claudin-5/6 pAb immunofluorescently stained L transfectants expressing claudin-5/TMVCF (C5L cells) and claudin-6 (C6L cells), whereas anti–claudin-6 pAb stained only C6L cells. Bar, 10 μm.
Construction of Expression Vectors and Transfection
To construct claudin-5/TMVCF or claudin-6 expression vectors with or without a FLAG-tag at their COOH termini, EcoRI sites were introduced at 3′ ends of claudin cDNAs by PCR, and amplified fragments were subcloned into pBluescript SK(−)–FLAG or SK(−). The inserts were excised by SaII-XbaI digestion followed by blunting with T4 polymerase, and then introduced into pCAGGSneodelEcoRI (Niwa et al. 1991), provided by Dr. J. Miyazaki (Osaka University, Osaka, Japan).
Mouse L cells were used for transfection. Aliquots of 1 μg of each expression vector were introduced into L cells in 1 ml of DMEM using Lipofectamine Plus (GIBCO BRL). After 24- or 48-h incubation, cells were replated and cultured in DMEM containing 10% FCS and 500 μg/ml of Geneticin (GIBCO BRL) to select stable transfectants.
Immunostaining
For whole-mount staining, mouse 12.5-d embryos were killed. Samples were pretreated by microwaving in PBS for 20 s and fixed in 4% paraformaldehyde/PBS for 30 min. They were dehydrated in methanol and bleached with 30% H2O2. Samples were then rehydrated, blocked with PBS-MT (0.2% Triton X-100 and 1% skimmed milk/PBS) and incubated overnight with primary antibodies followed by secondary antibodies. HRP-conjugated goat anti–rabbit Ig (Chemicon International, Inc.) was used as a secondary antibody. They were then washed with PBS-MT and PBS-T (0.2% Triton X-100/PBS), each for 5 h. Bound antibodies were visualized by incubating with 0.025% diaminobenzidene, 0.08% NiCl2, and 30% H2O2 in PBS-T.
Mouse brain, lung, kidney, and intestine were frozen using liquid nitrogen. Frozen sections ∼6 μm thick were cut on a cryostat, mounted on glass slides, air-dried, and fixed in 95% ethanol at 4°C for 30 min followed by 100% acetone at room temperature for 1 min. They were then rinsed in PBS containing 0.2% Triton X-100 for 15 min, blocked with 1% BSA/PBS for 15 min, and incubated with primary antibodies. After washing with PBS three times, samples were incubated for 30 min with secondary antibodies. Cy3-conjugated goat anti–rat Ig (Amersham Pharmacia Biotech) and Cy2-conjugated goat anti–rabbit Ig (Jackson ImmunoResearch Laboratories, Inc.) were used as secondary antibodies. Samples were washed three times with PBS, then mounted in 90% glycerol/PBS containing 0.1% paraphenylenediamine and 1% n-propylgalate. Specimens were observed using a Zeiss Axiophot photomicroscope (Carl Zeiss, Inc.).
SDS-PAGE and Immunoblotting
Lysates of Escherichia coli expressing GST–claudin fusion proteins (Morita et al. 1999a) were subjected to one-dimensional SDS-PAGE (12.5%) according to the method of Laemmli 1970, and gels were stained with Coomassie brilliant blue R-250. For immunoblotting, proteins were electrophoretically transferred from gels onto nitrocellulose membranes, which were then incubated with the first antibody. Bound antibodies were detected with biotinylated secondary antibodies and streptavidin-conjugated alkaline phosphatase (Amersham Pharmacia Biotech). Nitroblue tetrazolium and bromochloroindolyl phosphate were used as substrates for detection of alkaline phosphatase.
Freeze–Fracture Electron Microscopy
Immunoelectron microscopy to examine freeze–fracture replicas was performed as described (Fujimoto 1995). The mouse lung was cut into small pieces and quickly frozen in high pressure liquid nitrogen with an HPM 010 High Pressure Freezer (BAL-TEC). The frozen samples were fractured at −110°C and platinum-shadowed unidirectionally at an angle of 45° kin Balzers Freeze–Etch System (BAF 060; BAL-TEC). The samples were immersed in a sample lysis buffer containing 2.5% SDS, 10 mM Tris-HCl, and 0.6 M sucrose (pH 8.2) for 12 h at room temperature, and then replicas floating off the samples were washed with PBS. Under these conditions, integral membrane proteins were captured by replicas, and their cytoplasmic domains were accessible to antibodies. The replicas were incubated with anti–claudin-5/6 pAb for 60 min, then washed with PBS several times. They were then incubated with goat anti–rabbit Ig coupled to 10 nm gold (Amersham Pharmacia Biotech). The samples were washed with PBS, picked up on formvar-filmed grids, and examined in a JEOL 1200EX electron microscope at an accelerating voltage of 100 kV.
Results
Generation of Antibodies to Detect Claudin-5/TMVCF
The GST fusion protein with the cytoplasmic domain of claudin-5/TMVCF (see Fig. 1 A) was produced in E. coli and used as an antigen to generate specific pAbs in rabbits. Several pAbs that recognized claudin-5/TMVCF were obtained, but all of them cross-reacted with claudin-6 on immunoblotting (Fig. 1 B) as well as immunofluorescence microscopy (Fig. 1 C). As shown in Fig. 1 A, the COOH-terminal KNYV sequence was shared between claudin-5/TMVCF and claudin-6. The GST fusion protein with the cytoplasmic domain of claudin-6 lacking these four aa (GST–claudin-6Δ) was not detected by these pAbs (Fig. 1 B), indicating that they specifically recognized the COOH-terminal KNYV. These pAbs were then referred to as anti–claudin-5/6 pAbs. On the other hand, when the GST fusion protein with the cytoplasmic domain of claudin-6 was used as an antigen, several pAbs, which recognized claudin-6 but not claudin-5/TMVCF on immunoblotting (Fig. 1 B) as well as immunofluorescence microscopy (Fig. 1 C), were obtained (anti–claudin-6 pAb). As expected, these pAbs recognized GST–claudin-6Δ (Fig. 1 B). Therefore, to examine the expression and localization of claudin-5/TMVCF in various tissues, these anti–claudin-5/6 pAbs and anti–claudin-6 pAbs were used in combination; if some cells were anti–claudin-5/6 pAb-positive and anti–claudin-6 pAb-negative, we concluded that they expressed claudin-5/TMVCF. Fortunately, Northern blotting revealed that in most organs of adult mice the expression of claudin-6 was fairly restricted (Morita et al. 1999a).
Claudin-5/TMVCF in the Brain and Lung
We first examined the distribution of claudin-5/TMVCF in the brain, which does not contain epithelial cells but expressed claudin-5/TMVCF as detected by Northern blotting (Morita et al. 1999a). When 12.5-d mouse embryos were labeled by whole-mount immunostaining with anti–claudin-5/6 pAb, a characteristic tree-like staining pattern was detected in the head portion (Fig. 2, a and b). Anti–claudin-6 pAb gave no significant staining in the head portion (Fig. 2 c), and a pAb specific for PECAM, a specific marker for endothelial cells of blood vessels (Risau and Flamme 1995), showed a very similar staining pattern to anti–claudin-5/6 pAb (Fig. 2 d). These findings indicated that claudin-5/TMVCF was expressed exclusively in endothelial cells of all segments of blood vessels in the fetal brain.
Figure 2.
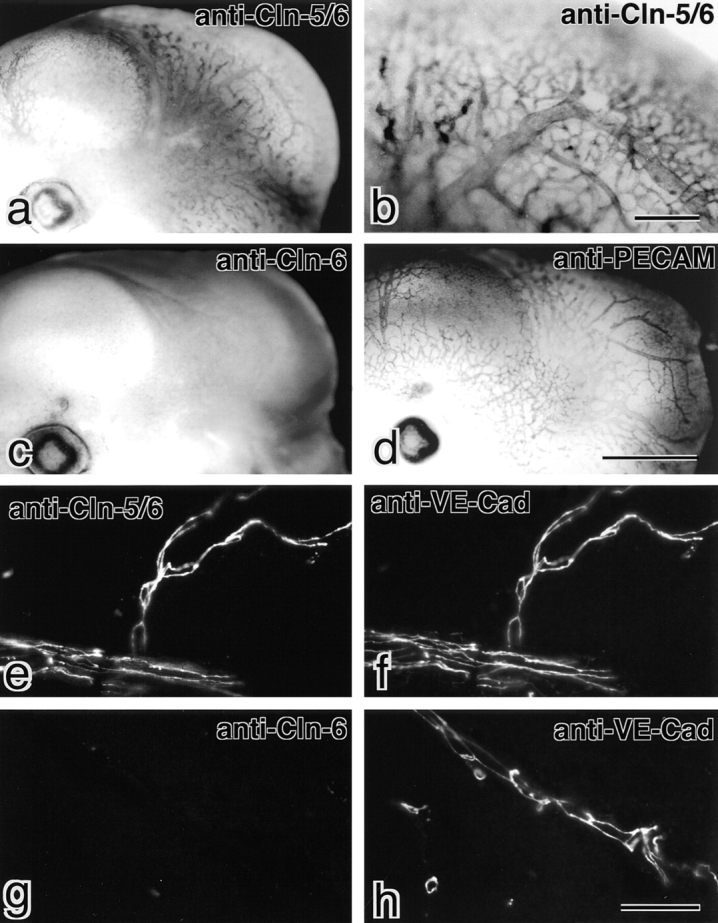
Exclusive expression of claudin-5/TMVCF in endothelial cells in the brain. (a–d) Whole-mount immunostaining. Mouse 12.5-d embryos were labeled with anti–claudin-5/6 pAb (a and b), anti–claudin-6 pAb (c), or anti-PECAM pAb (d). Both anti–claudin-5/6 pAb and anti-PECAM pAb showed similar tree-like staining of blood vessels in the head portion. In contrast, anti–claudin-6 pAb yielded no signals in the head portion. (e–h) Immunofluorescence staining. Frozen sections of adult mouse brain were double labeled with anti–claudin-5/6 pAb (e) and anti–VE-cadherin mAb (f) or anti–claudin-6-pAb (g) and anti–VE-cadherin mAb (h). Since anti–claudin-6 pAb yielded no signal at all, anti–claudin-5/6 pAb staining can be considered to represent the distribution of claudin–5/TMVCF. All blood vessels in the brain were VE-cadherin–positive, and claudin-5/TMVCF was precisely colocalized with VE-cadherin. Bars: 1 mm for a, c, and d (d); 0.2 mm (b); 40 μm for e–h (h). Cln, claudin; Cad, cadherin.
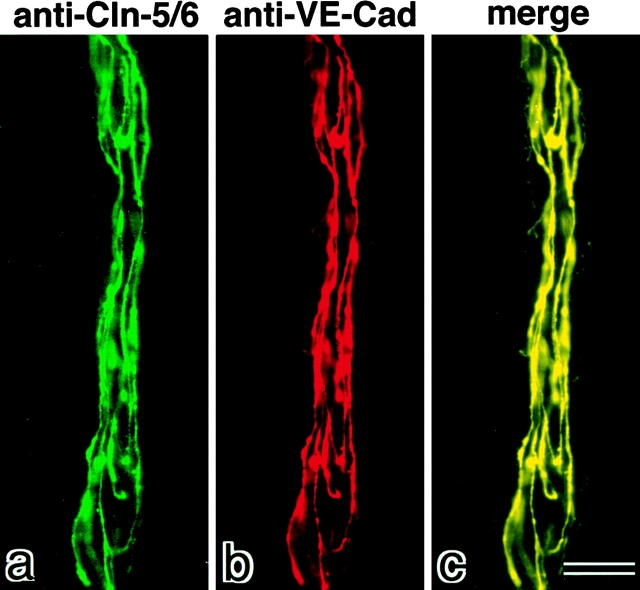
Frozen sections of adult brain were then immunofluorescently double stained with anti–claudin-5/6 pAb and anti–VE-cadherin mAb. VE-cadherin was reported to be specifically expressed in endothelial cells (Lampugnani et al. 1995). As shown in Fig. 2e and Fig. f, all blood vessels were exclusively costained with these antibodies. These anti–VE-cadherin mAb-positive endothelial cells were negative for staining with anti–claudin-6 pAb (Fig. 2g and Fig. h). Furthermore, at higher magnification, both claudin-5/TMVCF and VE-cadherin were shown to be precisely coconcentrated at cell–cell borders of endothelial cells of blood vessels (Fig. 3). Taken all together, we concluded that claudin-5/TMVCF is expressed and localized at cell–cell contact sites of endothelial cells of all segments of blood vessels in the adult brain.
Figure 3.
The precise colocalization of claudin-5/TMVCF and VE-cadherin at the cell–cell borders of endothelial cells in the brain. Blood vessels in frozen sections of adult mouse brain were double stained with anti–claudin-5/6 pAb (a, green) and anti–VE-cadherin mAb (b, red). Merged image (c) revealed that both staining patterns precisely overlapped. Anti–claudin-6 pAb yielded no signals (data not shown). Bar, 10 μm. Cln, claudin; Cad, cadherin.
Next, we examined the distribution of claudin-5/TMVCF in the lungs, which contained both epithelial and endothelial cells and expressed fairly large amounts of claudin-5/TMVCF as detected by Northern blotting (Morita et al. 1999a). When frozen sections of the lung were double stained with anti–claudin-5/6 pAb and anti–VE-cadherin mAb, both signals completely overlapped (Fig. 4, a–c). In contrast, on double staining with anti–claudin-5/6 pAb and antioccludin mAb, the two signals did not overlap at all (Fig. 4, d–f). Furthermore, anti–claudin-6 pAb gave no detectable signals (data not shown). Considering that occludin was expressed in epithelial cells but not in most of the endothelial cells in nonneuronal tissues, these findings indicated that claudin-5/TMVCF was expressed in endothelial cells of all segments of blood vessels but not in epithelial cells delineating the alveolar space in the lung. Furthermore, immunoreplica analysis with anti–claudin-5/6 pAb revealed that claudin-5/TMVCF was localized on the TJ strands of the endothelial cells of the lung (Fig. 4 g).
Figure 4.
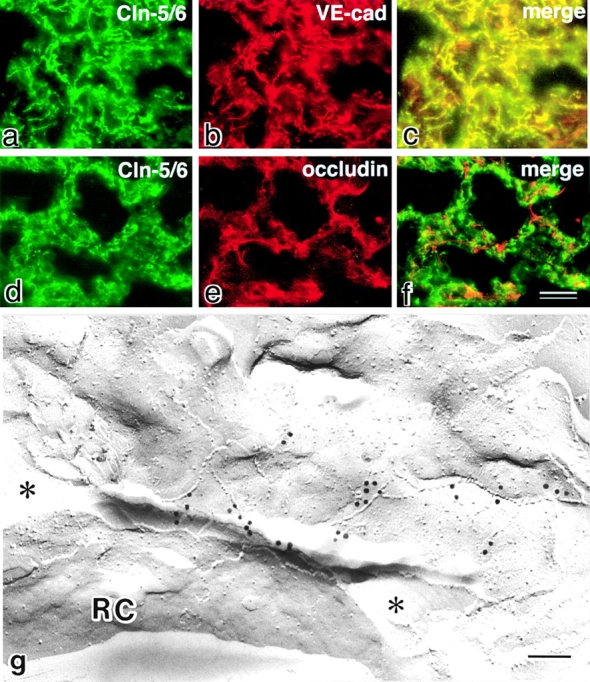
Localization of claudin-5/TMVCF in the lung. (a–f) Frozen sections of the lung were double stained with anti–claudin-5/6 pAb (a) and anti–VE-cadherin mAb (b), or anti–claudin-5/6 pAb (d) and antioccludin mAb (e). Since anti–claudin-6 pAb yielded no signals (data not shown), the anti–claudin-5/6 pAb staining can be considered to represent the distribution of claudin-5/TMVCF. Merged images (c and f) then revealed that claudin-5/TMVCF was precisely colocalized with VE-cadherin and that the claudin-5/TMVCF signals were complementary to the occludin signals. Considering that VE-cadherin and occludin were expressed exclusively in endothelial and epithelial cells, respectively, in the lungs, these findings indicated that the expression of claudin-5/TMVCF was restricted to endothelial cells. (g) Freeze–fracture replicas of the lung were labeled with anti–claudin-5/6 pAb. Note the specific labeling on the TJ strands of endothelial cells, which delineated the capillary lumen (indicated by asterisks). RC, erythrocytes; Cln, claudin; cad, cadherin. Bars: 20 μm in a–f (f); 100 nm (g).
Claudin-5/TMVCF in the Kidney and Other Organs
In the kidney, claudin-5/TMVCF did not appear to be expressed in endothelial cells of all segments of the blood vessels (Fig. 5). When frozen sections of the kidney cortex were doubly-stained with anti–claudin-5/6 pAb and antioccludin mAb, some blood vessels running in the connective tissues surrounding renal tubules were stained with anti–claudin-5/6 pAb but not with antioccludin mAb (Fig. 5, a and b). Instead, antioccludin mAb labeled TJs of distal tubules intensely (Fig. 5 b). Anti–claudin-6 pAb showed no significant signals (Fig. 5 e), indicating that only claudin-5/TMVCF was detected in the kidney by the anti–claudin-5/6 pAb. Judging from the density/number of the claudin-5/TMVCF–positive blood vessels, only some selected vessels appeared to be stained with anti–claudin-5/6 pAb. Frozen sections of the kidney were double stained with anti–claudin-5/6 pAb and anti–VE-cadherin mAb (Fig. 5c and Fig. d). Anti–VE-cadherin mAb stained numerous capillaries surrounding renal tubules and in glomeruli. These intertubular and glomerular capillaries were not stained by anti–claudin-5/6 pAb. Interestingly, afferent as well as efferent arterioles of glomeruli were reproducibly stained with anti–claudin-5/6 pAb (Fig. 5c and Fig. d). Furthermore, thicker arteries (probably interlobular arteries) but not veins were also intensely stained with anti–claudin-5/6 pAb (Fig. 5g and Fig. h). Taken together, we concluded that in the kidney, claudin-5/TMVCF constituted TJs only in endothelial cells of the arteries.
Figure 5.
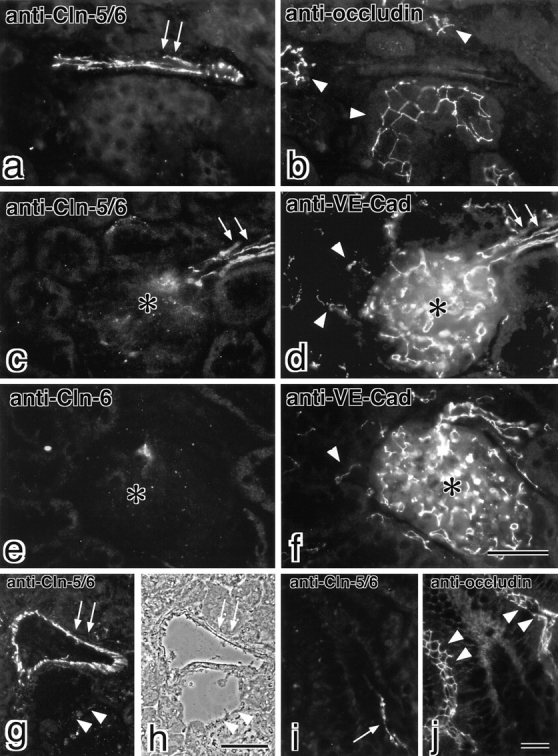
Localization of claudin-5/TMVCF in the kidney and the intestine. Frozen sections of the kidney (a–h) and the intestine (i and j) were double labeled with anti–claudin-5/6 pAb (a) and antioccludin mAb (b), anti–claudin-5/6 pAb (c) and anti–VE-cadherin mAb (d), anti–claudin-6 pAb (e) and anti–VE-cadherin mAb (f), or anti–claudin-5/6 pAb (i) and antioccludin mAb (j). Since anti–claudin-6 pAb yielded no signals in the kidney (e) and the intestine (data not shown), the anti–claudin-5/6 pAb staining can be considered to represent the distribution of claudin-5/TMVCF. Claudin-5/TMVCF appeared to be expressed in a subset of blood vessels (arrows in a and i) that were occludin-negative. Occludin was concentrated at TJs in distal tubules in the kidney (arrowheads in b) and intestinal epithelial cells (arrowheads in j). In the kidney, VE-cadherin–positive intertubular capillaries (arrowheads in d and f) and glomerular capillaries (asterisk in d and f) did not express claudin-5/TMVCF, but afferent and efferent arterioles (arrows in c and d) expressed both claudin-5/TMVCF and VE-cadherin. When transverse frozen sections of the thicker artery and vein (arrows and arrowheads, respectively, in g and h) of the kidney were stained with anti–claudin-5/6 pAb, only the artery was intensely stained (g). (h) Phase–contrast image. Bars: 40 μm for a–f (f); 70 μm in g and h (h); 40 μm in i and j (j). Cln, claudin; Cad, cadherin.
The expression and distribution of claudin-5/TMVCF were also examined in other organs, among them the intestine, skeletal muscle, skin, liver, testis. In these tissues claudin-5/TMVCF was specifically detected in endothelial cells in some segments of blood vessels, but not in epithelial cells. The endothelial cell–specific localization of claudin-5/TMVCF in the intestine was presented in Fig. 5i and Fig. j.
Reconstitution of Extracellular Face–associated TJ Strands in L Fibroblasts by Claudin-5/TMVCF
We reported previously that claudin-1 and -2 reconstituted TJ strands between adjacent transfectants when introduced into L fibroblasts (Furuse et al. 1998b). Then, we examined the ability of claudin-5/TMVCF to reconstitute TJ strands in L fibroblasts. When stable L transfectants expressing claudin-5/TMVCF were stained with anti–claudin-5/6 pAb, claudin-5/TMVCF was shown to be concentrated at cell–cell borders as planes, similar to claudin-1 and -2 (Fig. 6, a and b). Conventional freeze–fracture electron microscopy of glutaraldehyde-fixed L transfectants revealed that huge well-developed networks of TJ strands were formed in the cell–cell contact planes (Fig. 6 c). As shown previously in L transfectants expressing claudin-1 and -2 (Furuse et al. 1998b), claudin-1–induced strands were largely associated with the P-face as mostly continuous structures with vacant grooves at the E-face (P-face–associated TJs), whereas claudin-2–induced strands were discontinuous at the P-face with complementary grooves at the E-face that were occupied by chains of particles (E-face–associated TJs). As shown in Fig. 6 c, the claudin-5/TMVCF–induced TJs were an extreme case of the E-face–associated TJs; almost all particles were associated with the E-face, leaving particle-free ridges on the P-face (Fig. 6 c, insets).
Figure 6.
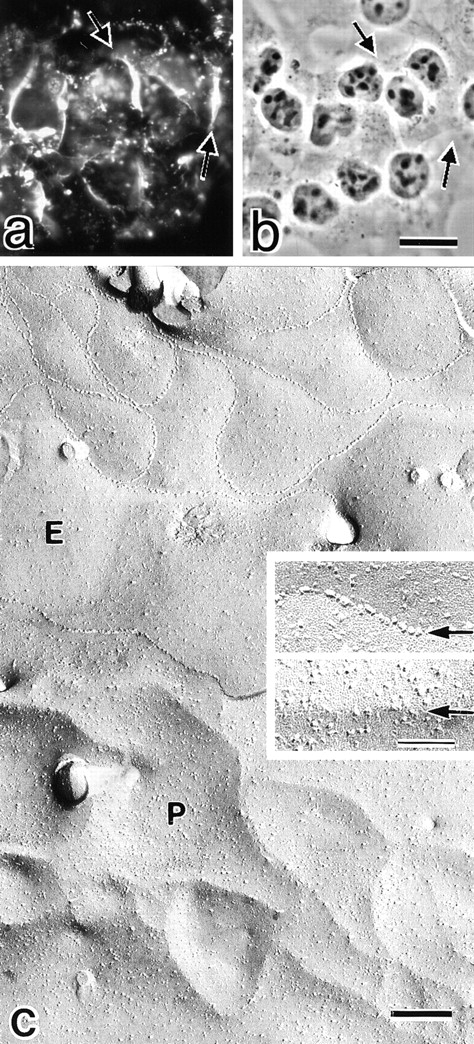
L transfectants expressing claudin-5/TMVCF. (a and b) Immunofluorescence (a) and corresponding phase–contrast images (b) of stable L transfectants expressing claudin-5/TMVCF. Cells were stained with anti–claudin-5/6 pAb. Expressed claudin-5/TMVCF was concentrated at cell–cell borders as planes (arrows). (c) Freeze–fracture images of cell–cell contact planes of stable L transfectants expressing claudin-5/TMVCF. Well-developed TJ strand/groove networks were reconstituted. In these reconstituted TJs, most TJ particles were recovered on the E-face; in the E-face (E), the grooves were completely occupied by chains of particles (arrow in upper inset), whereas only particle-free ridges were observed on the P-face (P) (arrow in lower inset). Bars: 15 μm in a and b (b); 200 nm (c); 50 nm (insets).
Discussion
TJs are thought to be involved in the barrier and fence functions in both epithelial and endothelial cells, but to date no differences have been reported in the molecular architecture of TJs between epithelial and endothelial cells (Rubin 1992; Schneeberger and Lynch 1992; Bowman et al. 1992; Goodenough 1999). In this study, we found that claudin-5/TMVCF was expressed specifically in endothelial cells, not in epithelial cells, and that this molecule constituted TJ strands in endothelial cells. The human TMVCF gene was originally found to be localized to chromosome 22q11, which is frequently deleted in velo-cardio-facial/DiGeorge syndrome patients (Sirotkin et al. 1997), and we proposed to designate it as claudin-5/TMVCF since it showed significant similarity to claudin-1 and -2 (Morita et al. 1999a). This gene was also identified as a membrane protein named MBEC1 that was expressed in the brain endothelial cells (Chen et al. 1998). In both studies, the concentration of the product of TMVCF/MBEC1 at TJs was not examined, probably due to the difficulty generating antibodies specific for this protein. We avoided this technical difficulty by generating and using anti–claudin-5/6 pAb and anti–claudin-6 pAb in combination. Immunofluorescence microscopy with these pAbs revealed that in the brain and the lung, the endothelial cells of all segments of blood vessels, which were positive in the anti–VE-cadherin mAb staining, expressed significant amounts of claudin-5/TMVCF. In contrast, in the kidney, veins and capillaries lacked the expression of claudin-5/TMVCF, and its expression was restricted to arteries. In addition, in all other tissues we examined, the expression of claudin-5/TMVCF was restricted to endothelial cells of some segments of blood vessels. Therefore, we concluded that claudin-5/TMVCF is an endothelial cell–specific component of TJ strands, but that in contrast to VE-cadherin (Lampugnani et al. 1995), claudin-5/TMVCF is not expressed in all types of endothelial cells.
Since it is widely accepted that TJs play central roles in the regulation of vascular permeability, the structure of endothelial TJs has been extensively examined, mainly by freeze–fracture electron microscopy (Dempsey and Bullivant 1973; Simionescu et al. 1975, Simionescu et al. 1976; Schneeberger 1982; Bowman et al. 1992). In general, in glutaraldehyde-fixed endothelial cells in nonneuronal tissues, TJ strands in endothelial cells were characterized by particle-free ridges on the P-face and continuous grooves at the E-face, which were densely occupied by chains of particles (E-face–associated TJs) (Dempsey and Bullivant 1973). Interestingly, the claudin-5/TMVCF–based TJ strands reconstituted in L transfectants showed the same morphological characteristics (see Fig. 6), favoring the notion that claudin-5/TMVCF is a major constituent of endothelial TJ strands of nonneuronal tissues in situ. Furthermore, in previous freeze–fracture studies, TJs were shown to be developed also in veins and capillaries in various tissues (Simionescu et al. 1975, Simionescu et al. 1976; Schneeberger 1982; Bowman et al. 1992). Therefore, some species of claudins other than claudin-5/TMVCF must be involved in the formation of TJ strands in veins and capillaries. To date, in addition to pAbs used in this study, pAbs specific for claudin-2, -3, -4, -8, -11, and -14 were available. Preliminary immunofluorescence microscopy with these pAbs showed that these claudins were not detected in endothelial cells in all of the organs we examined. Among the other claudin species (claudin-1, -7, -9, -10, -12, -13, and -15), claudin-1 is expected to be expressed in endothelial cells, since this claudin species is expressed rather ubiquitously, even in organs lacking epithelial tissues as shown by Northern blotting (Morita et al. 1999a). However, in contrast to claudin-5/TMVCF, claudin-1 appeared to be expressed also in epithelial cells, because this claudin species was first identified from hepatocytes and was expressed in large amounts in the liver and the kidney (Furuse et al. 1998a). Further identification and characterization of claudin species expressed in endothelial cells will be important in future studies.
Endothelial cells in the adult brain, which form the blood–brain barrier (BBB), are coupled by TJs of extremely low permeability that are more like those of epithelial barriers (Reese and Karnovsky 1967; Brightman and Reese 1969; Risau and Wolburg 1990; Rubin 1992). Despite the large numbers of in vivo studies that have been performed, the developmental timing of the formation and maturation of BBB in vivo is still controversial, but the TJs of brain endothelial cells are thought to be leaky in embryos (Møllgård and Saunders 1975; Farrell and Risau 1994). As shown in this study, claudin-5/TMVCF was clearly detected in endothelial cells of both the fetal and adult brain (see Fig. 2), suggesting that the formation and maturation of BBB is not attributable to the developmental changes in the expression level of claudin-5/TMVCF. TJ strands of endothelial cells in the fetal brain are E-face–associated similarly to those in nonneuronal tissues as discussed above, whereas those in the adult brain are P-face–associated (Wolburg et al. 1994; Kniesel et al. 1996). Therefore, it is tempting to speculate that during development the claudin-5/TMVCF–based, E-face–associated TJ strands are modified by addition of other claudin species or by some inside-out signaling, to be switched to the P-face–associated forms.
Although occludin and claudins were identified as components of epithelial TJ strands (Furuse et al. 1993; Ando-Akatsuka et al. 1996; Furuse et al. 1998a; Morita et al. 1999a), our knowledge of the molecular architecture of endothelial TJs is limited. Occludin was reported to be expressed in large amounts in brain endothelial cells, but was undetectable in most endothelial cells in nonneuronal tissues (Saitou et al. 1997; Hirase et al. 1997). Now that claudin-5/TMVCF has been identified as a specific component of endothelial TJ strands, it will be possible to examine and modulate the mechanism of regulation of vascular permeability in molecular terms. For example, vascular endothelial cell growth factor has been shown to elevate vascular permeability through binding to its tyrosine kinase–type receptors flt-1 and flk-1 (Risau et al. 1998; Gale and Yancopoulos 1999), and to cause disorganization of interendothelial junctions (Kevil et al. 1998). Thus, it would be intriguing to examine whether and how activation of these receptors modulates claudin-5/TMVCF. Furthermore, it will be important to analyze the possible involvement of claudin-5/TMVCF in vasogenic edema and inflammation in various pathological states. Further detailed analyses of claudin-5/TMVCF along these lines will lead to a better understanding of the molecular mechanisms behind vascular permeability.
Acknowledgments
We thank Drs. H. Yoshida and S.I. Nishikawa for their technical help with whole-mount immunostaining of mouse embryos. Our thanks are also due to all the members of our laboratory (Department of Cell Biology, Faculty of Medicine, Kyoto University) for helpful discussions. K. Morita thanks Prof. Y. Miyachi (Department of Dermatology, Faculty of Medicine, Kyoto University) for the opportunity to work in the Department of Cell Biology.
This study was supported in part by a Grant-in-Aid for Cancer Research and a Grant-in-Aid for Scientific Research (A) from the Ministry of Education, Science and Culture of Japan.
Note Added in Proof. Positional cloning has identified a new member of the claudin family (paracellin-1), mutations of which cause hereditary renal hypomagnesemia in humans (Simon, D.B., Y. Lu, K.A. Choate, H. Velazquez, E. Al-Sabban, M. Praga, G. Casari, A. Bettinelli, G. Colussi, J. Rodriguez-Soriano, et al. 1999. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 285:103–106). This new claudin species would be called claudin-16.
Footnotes
1.used in this paper: aa, amino acid(s); BBB, blood–brain barrier; E-face, extracellular face; GST, glutathione S-transferase; pAb, polyclonal antibody; PECAM, platelet endothelial cell adhesion molecule; P-face, protoplasmic face; TJ, tight junction; TMVCF, transmembrane protein deleted in velo-cardio-facial syndrome; VE-cadherin, vascular endothelial–cadherin
References
- Anderson J.M., Van Itallie C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- Ando-Akatsuka Y., Saitou M., Hirase T., Kishi M., Sakakibara A., Itoh M., Yonemura S., Furuse M., Tsukita Sh. Interspecies diversity of the occludin sequencecDNA cloning of human, mouse, dog, and rat–kangaroo homologues. J. Cell Biol. 1996;133:43–47. doi: 10.1083/jcb.133.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda M.S., González-Mariscal L., Matter K., Cereijido M., Anderson J.M. Assembly of the tight junctionthe role of diacylglycerol. J. Cell Biol. 1993;123:293–302. doi: 10.1083/jcb.123.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda M.S., Whitney J.A., Flores C., González S., Cereijido M., Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman P.D., du Bois M., Shivers R.R., Dorovini-Zis K. Endothelial tight junctions. In: Cereijido M., editor. Tight Junctions. CRC Press; London: 1992. pp. 305–320. [Google Scholar]
- Brightman M.W., Reese T.S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-H., Merzdorf C., Paul D.L., Goodenough D.A. COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos. J. Cell Biol. 1997;138:891–899. doi: 10.1083/jcb.138.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Zandonatti M., Jakubowski D., Fox H.S. Brain capillary endothelial cells express MBEC1, a protein that is related to the Clostridium perfringens enterotoxin receptors. Lab. Invest. 1998;78:353–363. [PubMed] [Google Scholar]
- Citi S., Sabanay H., Jakes R., Geiger B., Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988;333:272–276. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- Dempsey G.P., Bullivant S. Endothelial cell membranespolarity of particles as seen by freeze-fracturing. Science. 1973;179:190–192. doi: 10.1126/science.179.4069.190. [DOI] [PubMed] [Google Scholar]
- Farquhar M.G., Palade G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell C.L., Risau W. Normal and abnormal development of the blood-brain barrier. Microsc. Res. Tech. 1994;27:495–506. doi: 10.1002/jemt.1070270604. [DOI] [PubMed] [Google Scholar]
- Fujimoto K. Freeze–fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J. Cell Sci. 1995;108:3443–3449. doi: 10.1242/jcs.108.11.3443. [DOI] [PubMed] [Google Scholar]
- Furuse M., Hirase T., Itoh M., Nagafuchi A., Yonemura S., Sa. Tsukita, Tsukita Sh. Occludina novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Fujimoto K., Sato N., Hirase T., Sa. Tsukita, Tsukita Sh. Overexpression of occludin, a tight junction-associated integral membrane protein, induces the formation of intracellular multilamellar bodies bearing tight junction-like structures. J. Cell Sci. 1996;109:429–435. doi: 10.1242/jcs.109.2.429. [DOI] [PubMed] [Google Scholar]
- Furuse M., Fujita K., Hiiragi T., Fujimoto K., Tsukita Sh. Claudin-1 and -2novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin J. Cell Biol. 141 1998. 1539 1550a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Sasaki H., Fujimoto K., Tsukita Sh. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts J. Cell Biol. 143 1998. 391 401b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale N.W., Yancopoulos G.D. Growth factors acting via endothelial cell-specific receptor tyrosine kinasesVEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13:1055–1066. doi: 10.1101/gad.13.9.1055. [DOI] [PubMed] [Google Scholar]
- Goodenough D.A. Plugging the leaks. Proc. Natl. Acad. Sci. USA. 1999;96:319–321. doi: 10.1073/pnas.96.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B. Structure, biochemistry, and assembly of epithelial tight junctions. Am. J. Physiol. 1987;253:C749–C758. doi: 10.1152/ajpcell.1987.253.6.C749. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. Breaking through the tight junction barrier. J. Cell Biol. 1993;123:1631–1633. doi: 10.1083/jcb.123.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B., Lowenkopf T., Apatira D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc. Natl. Acad. Sci. USA. 1991;88:3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins J., Gu L., Wittchen E.S., Hibbard J., Stevenson B.R. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J. Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase T., Staddon J.M., Saitou M., Ando-Akatsuka Y., Itoh M., Furuse M., Fujimoto K., Tsukita Sh., Rubin L.L. Occludin as a possible determinant of tight junction permeability in endothelial cells. J. Cell Sci. 1997;110:1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- Keon B.H., Schäfer S., Kuhn C., Grund C., Franke W.W. Symplekin, a novel type of tight junction plaque protein. J. Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevil C.G., Payne D.K., Mire E., Alexander J.S. Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J. Biol. Chem. 1998;273:15099–15103. doi: 10.1074/jbc.273.24.15099. [DOI] [PubMed] [Google Scholar]
- Kniesel U., Risau W., Wolburg H. Development of blood-brain barrier tight junctions in the rat cortex. Dev. Brain Res. 1996;96:229–240. doi: 10.1016/0165-3806(96)00117-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lampugnani M.G., Corada M., Caveda L., Breviario F., Ayalon O., Geiger B., Dejana E. The molecular organization of endothelial cell to cell junctionsdifferential association of plakoglobin, β-catenin, and α-catenin with vascular endothelial cadherin (VE-cadherin) J. Cell Biol. 1995;129:203–217. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K.M., Skare I.B., Stankewich M.C., Furuse M., Tsukita Sh., Rogers R.A., Lynch R.D., Schneeberger E.E. Occludin is a functional component of the tight junction. J. Cell Sci. 1996;109:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- Møllgård K., Saunders N.R. Complex tight junctions of epithelial and endothelial cells in early foetal brain. J. Neurocytol. 1975;4:453–468. doi: 10.1007/BF01261375. [DOI] [PubMed] [Google Scholar]
- Morita K., Furuse M., Fujimoto K., Tsukita Sh. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands Proc. Natl. Acad. Sci. USA. 96 1999. 511 516a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., Sasaki H., Fujimoto K., Furuse M., Tsukita Sh. Claudin-11/OSP-based tight junctions in myelinated sheaths of oligodendrocytes and Sertoli cells in testis J. Cell Biol. 145 1999. 579 588b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H., Yamamura K., Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Reese T.J., Karnovsky M.J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W., Wolburg H. Development of the blood-brain barrier. Trends Neurosci. 1990;13:174–178. doi: 10.1016/0166-2236(90)90043-a. [DOI] [PubMed] [Google Scholar]
- Risau W., Flamme I. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- Risau W., Esser S., Engelhardt B. Differentiation of blood-brain barrier endothelial cells. Pathol. Biol. 1998;46:171–175. [PubMed] [Google Scholar]
- Rubin L.L. Endothelial cellsadhesion and tight junctions. Curr. Opin. Cell Biol. 1992;4:830–833. doi: 10.1016/0955-0674(92)90107-n. [DOI] [PubMed] [Google Scholar]
- Saitou M., Ando-Akatsuka Y., Itoh M., Furuse M., Inazawa J., Fujimoto K., Tsukita Sh. Mammalian occludin in epithelial cellsits expression and subcellular distribution. Eur. J. Cell Biol. 1997;73:222–231. [PubMed] [Google Scholar]
- Saitou M., Fujimoto K., Doi Y., Itoh M., Fujimoto T., Furuse M., Takano H., Noda T., Tsukita Sh. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J. Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger E.E. Structure of intercellular junctions in different segments of the intrapulmonary vasculature. Ann. NY Acad. Sci. 1982;384:54–63. doi: 10.1111/j.1749-6632.1982.tb21361.x. [DOI] [PubMed] [Google Scholar]
- Schneeberger E.E., Lynch R.D. Structure, function, and regulation of cellular tight junctions. Am. J. Physiol. 1992;262:L647–L661. doi: 10.1152/ajplung.1992.262.6.L647. [DOI] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Palade G.E. Segmental differentiation of cell junctions in the vascular endothelium. The microvasculature. J. Cell Biol. 1975;67:863–885. doi: 10.1083/jcb.67.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Palade G.E. Segmental differentiation of cell junctions in the vascular endothelium. Arteries and veins. J. Cell Biol. 1976;68:705–723. doi: 10.1083/jcb.68.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin H., Morrow B., Saint-Jore B., Puech A., Das Gupta R., Patanjali S.R., Skoultchi A., Weissman S.M., Kucherlapati R. Identification, characterization, and precise mapping of a human gene encoding a novel membrane-spanning protein from the 22q11 region deleted in velo-cardio-facial syndrome. Genomics. 1997;42:245–251. doi: 10.1006/geno.1997.4734. [DOI] [PubMed] [Google Scholar]
- Spring K. Routes and mechanism of fluid transport by epithelia. Annu. Rev. Physiol. 1998;60:105–119. doi: 10.1146/annurev.physiol.60.1.105. [DOI] [PubMed] [Google Scholar]
- Staehelin L.A. Further observations on the fine structure of freeze-cleaved tight junctions. J. Cell Sci. 1973;13:763–786. doi: 10.1242/jcs.13.3.763. [DOI] [PubMed] [Google Scholar]
- Staehelin L.A. Structure and function of intercellular junctions. Int. Rev. Cytol. 1974;39:191–283. doi: 10.1016/s0074-7696(08)60940-7. [DOI] [PubMed] [Google Scholar]
- Stevenson B.R., Siliciano J.D., Mooseker M.S., Goodenough D.A. Identification of ZO-1a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita Sh., Furuse M. Occludin and claudins in tight junction strandsleading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- Van Itallie C.M., Anderson J.M. Occludin confers adhesiveness when expressed in fibroblasts. J. Cell Sci. 1997;110:1113–1121. doi: 10.1242/jcs.110.9.1113. [DOI] [PubMed] [Google Scholar]
- Wolburg H., Neuhaus J., Kniesel U., Krauss B., Schmid E.M., Öcalan M., Farrell C., Risau W. Modulation of tight junction structure in blood-brain barrier endothelial cells. Effects of tissue culture, second messengers and cocultured astrocytes. J. Cell Sci. 1994;107:1347–1357. doi: 10.1242/jcs.107.5.1347. [DOI] [PubMed] [Google Scholar]
- Wong V., Gumbiner B.M. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J. Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap A.S., Mullin J.M., Stevenson B.R. Molecular analysis of tight junction physiologyinsights and paradoxes. J. Membr. Biol. 1998;163:159–167. doi: 10.1007/s002329900380. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Saitoh T., Minase T., Sawada N., Enomoto K., Mori M. Monoclonal antibody 7H6 reacts with a novel tight junction-associated protein distinct from ZO-1, cingulin, and ZO-2. J. Cell Biol. 1993;120:477–483. doi: 10.1083/jcb.120.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]