Abstract
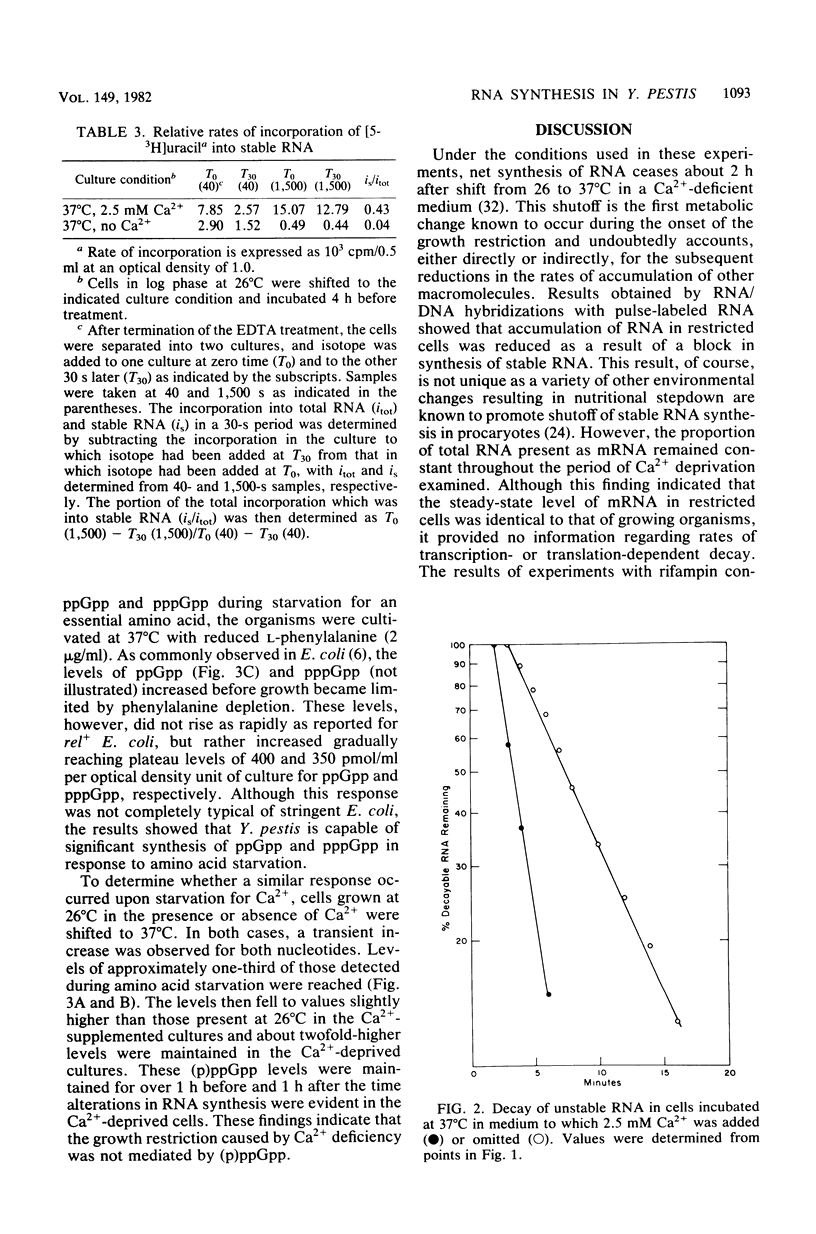
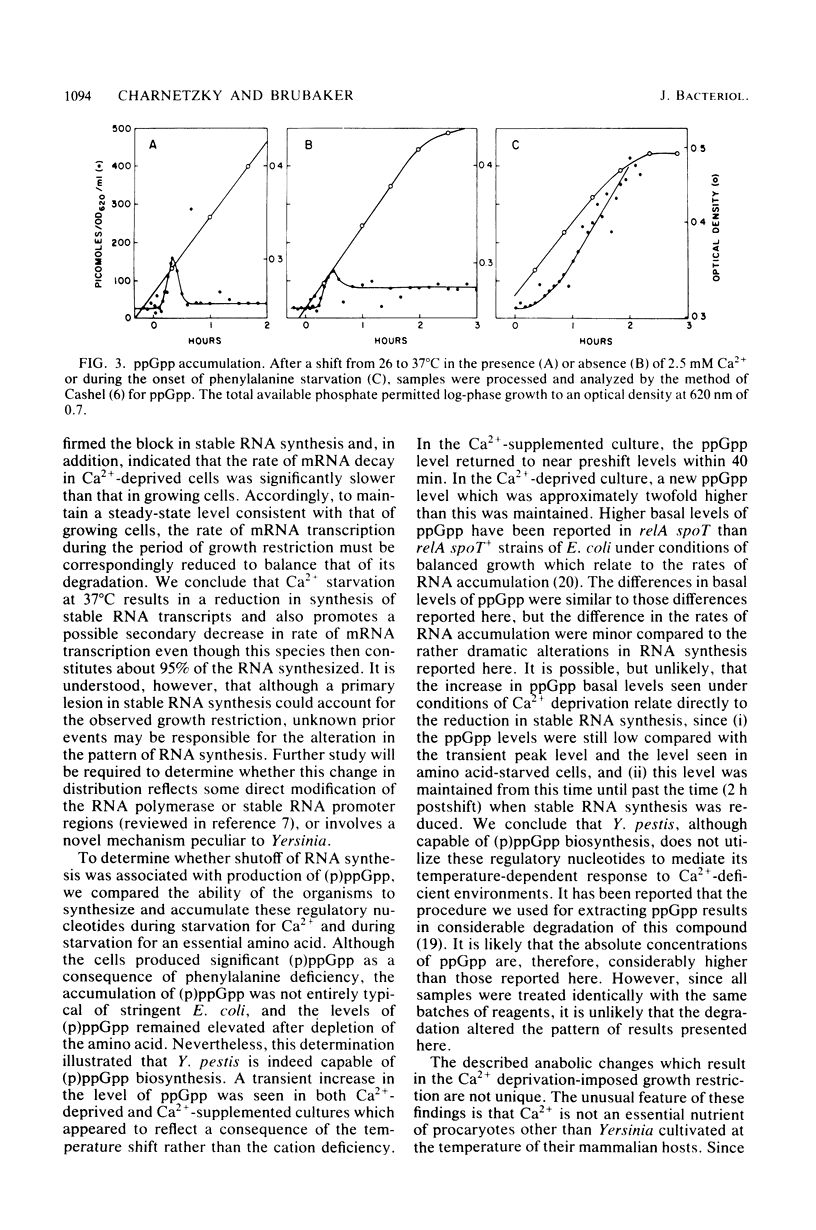
Yersinia pestis requires 2.5 mM Ca2+ for growth at 37°C but not at 26°C. After a shift from 26 to 37°C in a Ca2+-deficient medium, an ordered series of metabolic alterations occur which result in transition from a growing cell to a viable but non-proliferating cell. The earliest known alteration in normal metabolism associated with this transition is a termination of net RNA synthesis. Competitive RNA/DNA hybridizations with uniformly labeled RNA and stable RNA competitor indicated identical mRNA to stable RNA ratios in growing cells and non-proliferating Ca2+-deprived cells. Similar hybridizations with pulse-labeled RNA demonstrated that growing cells synthesized 57% mRNA, 37% rRNA, and 5% tRNA, whereas Ca2+-deprived cells synthesized 95% mRNA, 4.7% rRNA, and 0.7% tRNA. After addition of radioactive uracil and rifampin to growing and Ca2+-deprived cells, decay of approximately 40 and 90% of the newly synthesized RNA was found for growing and Ca2+-deprived cells, respectively. The half-life of the mRNA was found to be 1.5 min for growing cells and 4.5 min for Ca2+-deprived cells. Y. pestis elicited increases in the levels of guanosine tetraphosphate and guanosine pentaphosphate in response to amino acid deprivation and yielded transient increases in the levels of these phosphorylated nucleotides after a shift from 26 to 37°C. These increases were independent of Ca2+ availability and preceded the alteration in RNA synthesis by more than 1 h. The levels of these phosphorylated nucleotides then stabilized at about 80 and 40 pmol for Ca2+-deprived and Ca2+-supplemented cultures, respectively, and did not increase further in the Ca2+-deprived culture at the time corresponding to the reduction in stable RNA synthesis. These findings indicate that the early lesion in RNA synthesis associated with the growth restriction of Ca2+-deprived Y. pestis reflects a block in stable RNA synthesis and that this effect is not mediated by guanosine tetraphosphate or guanosine pentaphosphate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beveridge T. J., Murray R. G. Uptake and retention of metals by cell walls of Bacillus subtilis. J Bacteriol. 1976 Sep;127(3):1502–1518. doi: 10.1128/jb.127.3.1502-1518.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer H., Berry L., Dennis P. P. Regulation of ribonucleic acid synthesis in Escherichia coli B-r: an analysis of a shift-up. II. Fraction of RNA polymerase engaged in the synthesis of stable RNA at different steady-state growth rates. J Mol Biol. 1973 Mar 25;75(1):161–179. doi: 10.1016/0022-2836(73)90536-6. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. Interconversion of Purine Mononucleotides in Pasteurella pestis. Infect Immun. 1970 May;1(5):446–454. doi: 10.1128/iai.1.5.446-454.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R. The genus Yersinia: biochemistry and genetics of virulence. Curr Top Microbiol Immunol. 1972;57:111–158. doi: 10.1007/978-3-642-65297-4_4. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Substructure and in vitro assembly of the outer, structured layer of Spirillum serpens. J Bacteriol. 1976 Jan;125(1):290–299. doi: 10.1128/jb.125.1.290-299.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Dennis P. P., Bremer H. A method for determination of the synthesis rate of stable and unstable ribonucleic acid in Escherichia coli. Anal Biochem. 1973 Dec;56(2):489–501. doi: 10.1016/0003-2697(73)90216-9. [DOI] [PubMed] [Google Scholar]
- Dias F. F., Okrend H., Dondero N. C. Calcium nutrition of Sphaerotilus growing in a continuous-flow apparatus. Appl Microbiol. 1968 Sep;16(9):1364–1369. doi: 10.1128/am.16.9.1364-1369.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber D. M., Brubaker R. R. Plasmids in Yersinia pestis. Infect Immun. 1981 Feb;31(2):839–841. doi: 10.1128/iai.31.2.839-841.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Gillespie S., Gillespie D. Ribonucleic acid-deoxyribonucleic acid hybridization in aqueous solutions and in solutions containing formamide. Biochem J. 1971 Nov;125(2):481–487. doi: 10.1042/bj1250481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., KUPFERBERG L. L., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. III. Effects of calcium ions on the growth of virulent and avirulent strains of Pasteurella pestis. J Bacteriol. 1959 Mar;77(3):317–321. doi: 10.1128/jb.77.3.317-321.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. J., Yang G. C., Little R. V., Brubaker R. R. Effect of Ca2+ on morphology and division of Yersinia pestis. Infect Immun. 1974 Jun;9(6):1105–1113. doi: 10.1128/iai.9.6.1105-1113.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennel D. Titration of the gene sites on DNA by DNA-RNA hybridization. II. The Escherichia coli chromosome. J Mol Biol. 1968 May 28;34(1):85–103. doi: 10.1016/0022-2836(68)90236-2. [DOI] [PubMed] [Google Scholar]
- Kennell D., Bicknell I. Decay of messenger ribonucleic acid from the lactose operon of Escherichia coli as a function of growth temperature. J Mol Biol. 1973 Feb 15;74(1):21–31. doi: 10.1016/0022-2836(73)90351-3. [DOI] [PubMed] [Google Scholar]
- Kojima M., Suda S., Hotta S., Hamada K. Induction of pleomorphy and calcium ion deficiency in Lactobacillus bifidus. J Bacteriol. 1970 Apr;102(1):217–220. doi: 10.1128/jb.102.1.217-220.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWTON W. D., ERDMAN R. L., SURGALLA M. J. BIOSYNTHESIS AND PURIFICATION OF V AND W ANTIGEN IN PASTEURELLA PESTIS. J Immunol. 1963 Aug;91:179–184. doi: 10.21236/ad0299868. [DOI] [PubMed] [Google Scholar]
- Lagosky P. A., Chang F. N. Influence of amino acid starvation on guanosine 5'-diphosphate 3'-diphosphate basal-level synthesis in Escherichia coli. J Bacteriol. 1980 Nov;144(2):499–508. doi: 10.1128/jb.144.2.499-508.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagosky P. A., Chang F. N. The extraction of guanosine 5'-diphosphate, 3'-diphosphate (ppGpp) from Escherichia coli using low pH reagents: a reevaluation. Biochem Biophys Res Commun. 1978 Oct 30;84(4):1016–1024. doi: 10.1016/0006-291x(78)91685-6. [DOI] [PubMed] [Google Scholar]
- Moore R. L., McCarthy B. J. Comparative study of ribosomal ribonucleic acid cistrons in enterobacteria and myxobacteria. J Bacteriol. 1967 Oct;94(4):1066–1074. doi: 10.1128/jb.94.4.1066-1074.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Sadoff H. L. Relationship between calcium and uroinic acids in the encystment of Azotobacter vinelandii. J Bacteriol. 1975 Apr;122(1):145–151. doi: 10.1128/jb.122.1.145-151.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pato M. L., Bennett P. M., von Meyenburg K. Messenger ribonucleic acid synthesis and degradation in Escherichia coli during inhibition of translation. J Bacteriol. 1973 Nov;116(2):710–718. doi: 10.1128/jb.116.2.710-718.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz H. The importance of carboxyl groups for the biological activity of acyl carrier protein of Escherichia coli. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1446–1453. doi: 10.1016/s0006-291x(72)80139-6. [DOI] [PubMed] [Google Scholar]
- Silver S., Toth K., Scribner H. Facilitated transport of calcium by cells and subcellular membranes of Bacillus subtilis and Escherichia coli. J Bacteriol. 1975 Jun;122(3):880–885. doi: 10.1128/jb.122.3.880-885.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellen J. E., Raj H. D. Morphogenesis and fine structure of Leucothrix mucor and effects of calcium deficiency. J Bacteriol. 1970 Jan;101(1):240–249. doi: 10.1128/jb.101.1.240-249.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G. C., Brubaker R. R. Effect of ca on the synthesis of deoxyribonucleic Acid in virulent and avirulent yersinia. Infect Immun. 1971 Jan;3(1):59–65. doi: 10.1128/iai.3.1.59-65.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan D., Shen V. Stability of ribosomal and transfer ribonucleic acid in Escherichia coli B/r after treatment with ethylenedinitrilotetraacetic acid and rifampicin. J Bacteriol. 1975 May;122(2):425–432. doi: 10.1128/jb.122.2.425-432.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahorchak R. J., Charnetzky W. T., Little R. V., Brubaker R. R. Consequences of Ca2+ deficiency on macromolecular synthesis and adenylate energy charge in Yersinia pestis. J Bacteriol. 1979 Sep;139(3):792–799. doi: 10.1128/jb.139.3.792-799.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



