Abstract
Replication-defective adenoviral (RDAd) vectors can be generated at high titers and infect both dividing and nondividing cells. Long term expression in the transduced tissue, however, has been a problem because of the cellular immune responses against the infected cells. We demonstrate that mice injected with RDAd vectors containing mouse leptin gene reduce food intake and lose weight for only 7 to 10 days. Splenocytes obtained from infected mice are able to lyse target cells infected with RDAd vectors. Surprisingly, target cells infected with psoralen-treated, UV-crosslinked, biologically inactive RDAd also were lysed efficiently by the effector cells. Furthermore, splenocytes obtained from mice injected with inactive RDAd vectors efficiently lysed target cells infected with RDAd vectors. Whether RDAd vectors were injected i.m. or i.v. or through an i.p. route, the extent of lysis was similar. We propose that cells infected with RDAd vectors present antigens for recognition by class 1 major histocompatibility complex-restricted cytotoxic T lymphocytes by a mechanism that does not require viral replication or de novo protein synthesis. These results should prompt reevaluation of the use of RDAd vectors for gene therapy when long-term expression is required.
Successful gene therapy approaches require not only efficient transfer of the therapeutic gene to desired somatic tissues but also sustained expression of the therapeutic biomolecules. A wide variety of delivery vehicles have been used but each method has some major limitation (1). The most extensively used vector system uses retroviruses because, on infection, the proviral DNA containing the foreign gene is integrated into the host chromosome. Unfortunately, infection by retroviral vectors requires the cells to divide, which limits their application for in vivo gene delivery (1, 2). The adeno-associated viral vectors can infect nondividing cells and can integrate in the chromosome but have limited size capacity for the transgene. Additionally, large-scale production of adeno-associated viral vectors still faces considerable challenges. Recently, lentiviral vectors have been generated that have the ability to infect dividing and nondividing cells (3). Furthermore, expression of the transgene can be observed in vivo for several months (4, 5).
Recombinant adenoviruses, by virtue of their ability to transfer genes efficiently in vivo, have commanded considerable attention (6–8). Enthusiasm for widespread use of adenoviral vectors, however, has been tempered by the associated cellular and humoral immune response problems (9, 10). The cellular immunity is in part caused by activation of CD8+ lymphocytes against cells producing adenoviral proteins and the product of the transgene (9–15). Because the recombinant adenoviral vectors were devoid of E1A and E1B genes, it was a surprise that late viral mRNAs and proteins could be detected in the infected cell (9, 10). Clearly, replication-defective recombinant adenoviral (RDAd) vectors can bypass the requirement for viral transactivating (E1A) protein, particularly when infected at a high multiplicity of infection (moi) (16, 17). Furthermore, animals injected with agents that block cytotoxic T lymphocyte (CTL) formation (e.g., immunosuppressive drugs or CTLA4Ig) show sustained production of the transgene (10, 18, 19). Therefore, several investigators have argued that, if recombinant adenoviruses can be debilitated further—for instance, by the removal of E2A gene (DNA binding protein) or the generation of “gutless” vectors (removal of all viral coding region), expression of the transgene may be sustained (12, 15, 18). Accordingly, infection with E2A deleted or gutless recombinant adenoviruses showed expression for longer time periods (20–31). Before investing extensive efforts to generate debilitated adenoviruses that are unable to synthesize viral proteins de novo and therefore may not induce CTLs, we asked the question of whether psoralen-treated, UV-crosslinked, biologically inactive recombinant adenoviruses will generate CTLs on in vivo injection. Additionally, by using RDAd vectors containing mouse factor IX or mouse leptin cDNA, we investigated the previous proposal (32) that CTL response to RDAd virus infected cells was directed against the foreign (nonself) transgene product.
Here, we demonstrate that (i) CD8+ and CD4+ lymphocytes can be detected at the site of injection in muscle with biologically active or psoralen treated UV-crosslinked inactive RDAd vectors; (ii) i.p. injection of RDAd vectors expressing mouse leptin gene in obese (OB/OB) mice leads to a dramatic weight loss and reduced food intake for 4–8 days; however, both the weight and food intake gradually returned to pretreatment level; (iii) both murine and canine factor IX RDAd vectors, on injection in hind leg muscle of either BALB/c or B6 mice, recruited CD8+ and CD4+ lymphocytes at the site of injection, thereby ruling out the origin of the transgene as the sole basis of cellular immunity; and (iv) CTL assays show that splenocytes from mice transduced with either biologically active or inactive RDAd vectors can lyse target cells infected with a variety of RDAd vectors. We conclude that RDAd vectors do not require either viral replication or de novo viral protein synthesis to induce CTLs capable of lysing infected cells.
MATERIALS AND METHODS
Animal Procedures.
Female C57BL/6J OB/OB, female wild-type C57BL/6J, and female BALB/c mice were obtained from The Jackson Laboratory and were handled in accordance with the institutional guidelines of the Salk Institute. The OB/OB mice and the wild-type C57B6 mice were caged individually. 1 × 109 units RDAd vectors expressing either murine factor IX or murine leptin were administered to 12-week-old mice by i.p. injection. The mice were monitored for body weight and food consumption every 2 days.
For i.m. administration, active or psoralen-inactivated RDAd vectors were diluted in 100 μl of PBS and were injected into the hind leg muscles of anesthetized mice. To facilitate later isolation of the injected muscles, carbon black dispersion solution (Faber-Castell, Newark, NJ) was added to the injected vector solution before injection. For i.v. administration, purified RDAd vectors were diluted in 100 ml of PBS and were injected into the tail vein. The levels of canine factor IX were determined in mouse plasma, and culture media were determined by ELISA (32).
Adenoviral Vectors.
The ob adenoviral vector containing mouse leptin cDNA was a gift from Izumo Saito (Univ. of Tokyo). The mouse leptin cDNA [nucleotides 109–621 (33)] was expressed under the control of the CAG promoter as described (34). The construction of the RDAd AxCANLacZ that expresses the nuclear localized LacZ under the control of the CAG promoter and the Adex100 vector from which no transgene is expressed has been described (34, 35). RDAds expressing either canine or murine factor IX were constructed as described (10, 36).
Inactivation of RDAd Vectors.
The protocol used in this study to inactivate RDAd vectors was based on a method described by Cotten et al. (37). Purified RDAd vectors were diluted in PBS solution containing 50% glycerol and 250 μg/ml 8-methoxypsoralen. The diluted vectors were UV irradiated for 1h by Stratagene Eagleye UV transilluminator, which was placed in a distance of 3 cm from the coverless 3.5-cm plate containing the RDAd solution. The irradiated vector was kept on ice throughout the procedure. Psoralen was removed by two 12-h rounds of dialysis against PBS solution containing 10% glycerol. The efficiency of inactivation was determined by infecting 105 HeLa cells with the inactivated RDAd vectors at a moi of 50. The expression of the inactivated LacZ and canine factor IX transgene in the transduced HeLa cells was assayed by β-gal staining and factor IX ELISA, respectively.
Production of Effector Cells.
Mice were infected with 109 units of the indicated virus 3 weeks before being killed. Spleen cells were prepared as single cell suspensions in complete RPMI (RPMI 1640 supplemented with 10% fetal calf serum, 25 mM Hepes, 2 mM glutamine, 5 × 10−5 M β-mercaptoethanol, and 50 μg/ml gentamicin) at a concentration of 6 × 106 cells per milliliter. Stimulators were irradiated with syngeneic spleen cells (3,000 rads) infected with 1 × 108 units of RDAd vector per 107 cells and were resuspended at 6 × 106 cells per milliliter. Mixtures of 1 ml each, stimulators and responders, were co-cultured in 24-well tissue culture plates for 6 days at 37°C in a humidified atmosphere of 5% CO2.
Cytotoxicity Assay.
Target cells were incubated with 200 μC of 51Cγ of sodium chromate for 1 h. Viral-infected targets also received the indicated viruses (moi of 50). Target cells were washed three times, were resuspended in complete RPMI, and were seeded into 96-well plates at 104 cells per well in 100 μl complete RPMI medium 1640. Effector CTL were harvested and washed three times in complete RPMI and were seeded into duplicate wells containing the appropriate target cells at various E:T cell ratios, making a final volume of 200 μl. Plates were incubated at 37°C in a humidified incubator with 5% vol/vol CO2 for 6 h. Plates were centrifuged, and 100 μl of supernatant was removed from each well to assess isotope release by using a gamma-irradiation counter. The percent specific lysis was determined by the formula: percent specific lysis equals (sample release minus spontaneous release over maximum release minus spontaneous release) times 100. All cytolytic analyses described in this work were performed at least three times.
Immunohistochemistry.
Transduced muscle tissue was identified by charcoal staining and was isolated from the hind legs of injected mice. Isolated tissue was frozen in a freezing medium without prior chemical fixation and was sectioned serially on a freezing microtome. The presence of CD+4 CD+8 lymphocytes in the transduced tissue was determined by immunohistochemistry. The sections were blocked in 10% normal goat serum for 30 min, were washed three times with PBS, and were incubated with rat anti-mouse CD8 antibodies.
RESULTS
RDAd Vectors Expressing Self or Foreign Transgene Recruit CD+4 and CD+8 Cells To Transduced Tissues.
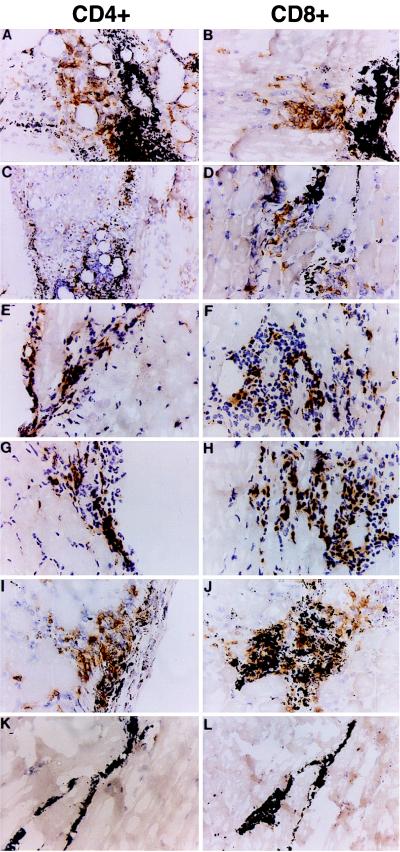
To study the role of transgene product in the induction of the immune response against RDAd transduced cells, we injected 109 units of RDAd expressing either murine (self) or canine (foreign) factor IX genes into the hind leg muscle of BALB/c mice. Additionally C57BL/6 (B6) and obese (OB/OB) mice were injected with RDAd expressing murine leptin gene. The animals were killed 5 or 12 days after injection, and the transduced muscle tissues were immunostained for CD4+ and CD8+ cells. As shown in Fig. 1, lymphocyte infiltration at the injection site was independent of the nature of the transgene. Similar levels of lymphocyte infiltration were observed when BALB/c mice were injected with RDAd expressing either canine or murine factor IX (Fig. 1 A–D). No differences in lymphocyte infiltration were observed in the injected muscles of wild-type B6 or OB/OB (B6 background) mice after transduction by RDAd viruses expressing murine leptin gene (Fig. 1 E–H). These data indicate that the recruitment of lymphocytes at the site of injection in the transduced tissues after infection with RDAd vectors is independent of the nature or origin of the transgene as well as strains of mice.
Figure 1.
Lymphocyte infiltration at the injection site. Histological sections taken from the hind-leg muscles of BALB/c (A–D and I–L), C57/black (E and F), and OB/OB (G and H) mice 5 days after being injected i.m. with 109 units of Ad CMV-mF9 (A and B), Ad CMV-cF9 (C and D), Ad CAG-mLeptin (G–J), or 100 mg of BSA (K and L). The muscle tissue was harvested and snap frozen in OCT. Cryosections were cut and stained with primary antibodies to murine L3T4 (CF4) and Ly2 (CD8) (PharMingen), followed by a biotinylated secondary antibody and an avidin-biotin-peroxidase complex (Vector Laboratories) and then were visualized by using diaminobenzidine as a chromogen. The black grains in all of the sections are the charcoal dye that marked the injection sites.
RDAd Transduced Cells Are Lysed by Infiltrating Lymphocytes.
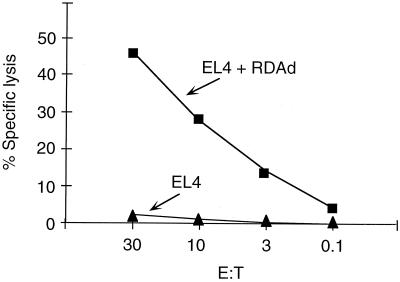
Although the RDAd transduced tissues were infiltrated with CD4+ and CD8+ lymphocytes independent of the nature of the transgenes being expressed, it was not clear whether the infiltrating lymphocytes can lyse the transduced target cells. We injected B6 mice i.m. with 109 units of RDAd vector expressing murine leptin gene. Splenocytes obtained from the mice 3 weeks after injection were stimulated in vitro with RDAd virus-infected cells. The effector cells were used in chromium release assay that measured CTL response against histocompatibility complex-matched target EL4 cells. The target cells were infected with RDAd vectors containing transgene. Fig. 2 shows that splenocytes from RDAd-murine leptin-infected B6 mice were highly effective in lysing RDAd transduced target cells. Furthermore, target cells expressing either murine leptin or mouse or canine factor IX also lysed as efficiently (data not shown). Because target cells transduced with RDAd vectors containing no transgene were lysed, we propose that the cellular immune response was elicited mainly against viral antigens as has been described (refs. 18 and 19). Although the splenocytes obtained from the RDAd injected mice demonstrated CTL activity against transduced target cells in vitro, we could not estimate the extent of the immune response in vivo.
Figure 2.
CTL assay in mice injected with Leptin-RDAd. C57/black (B6) mice were immunized i.m. with 109 units of Ad CAG-mLeptin 3 weeks before death. Splenocytes were removed and cultured with homologous, irradiated antigen-presenting cells infected with Ad CAG-mLeptin. On day 6, cells were assayed for the presence of adenovirus-specific CTL by lysis of 51Cr-labeled EL4 cells (▴) andEL4 cells infected with RDAd (without transgene) (■).
Loss of Biological Effect in Murine Leptin-RDAd-Infected OB/OB Mice.
To evaluate the extent of the immune response against RDAd transduced cells in vivo, we measured the biological effects in OB/OB mice after infection with murine leptin-RDAd vector. Five OB/OB mice were injected i.p. with 109 units of RDAd vector-expressing murine leptin gene under the control of the CAG promoter. As a control, we injected five OB/OB mice with 109 units of RDAd vectors expressing the murine factor IX. Both the food intake and the weight of the mice were measured every 2 days and served as an indicator of biologic activity of leptin gene product. There was no significant change in food intake and body weight of the mice injected with murine factor IX-expressing RDAd vectors (Fig. 3 B and D) although mice injected with RDAd vectors expressing leptin exhibited dramatic changes in both food intake and body weight (Fig. 3 A and C). During the first 4 days after injection, dramatic decline in food intake of all mice was observed (Fig. 3A). Two of the five mice injected with the leptin-RDAd vectors did not eat at all whereas the food consumption of the other three mice was <10% of their regular food intake. No dramatic changes in food intake were observed between day 4 and day 8 after injection, after which continuous increase in food intake was observed, eventually returning to pretreatment levels between 2 to 3 weeks after injection. The lack of food intake was reflected in the changes in body weight because all mice lost ≈30% of their body weight during the first 10 days (Fig. 3C). However, after 10 days, a steady increase in body weight could be observed (Fig. 3C). Within a month and a half, all of the mice reached their pretreatment body weight. These results, combined with the results of lymphocyte migration and CTL assays (Fig. 1, 2), suggest that the cellular immune response induced after infection with RDAd vectors in immunocompetent mice was efficient enough to eliminate most of the transduced cells and, consequently, the loss of expression of the leptin gene product. Although we have no formal proof that lack of expression of the leptin gene is caused by the shut off of transcription, it is very unlikely because in nude mice expression of foreign gene product was observed for >360 days (10).
Figure 3.
Expression in mice transduced with leptin-RDAd. Transient decrease in food intake (A and B) and body weight (C and D) in OB/OB mice after in vivo leptin gene transduction. OB/OB mice were injected i.p. with either 109 units of Ad CAG-mLeptin (A and C; OB2-OB5 mice) or Ad CMV-mF9 at 109 units (B and D; OB7-OB9 mice). Food intake and body weight of individually caged mice were measured every 48 h.
De Novo Synthesis of Viral Proteins Is Not Necessary for Eliciting the Immune Response.
The CTLs that eliminated RDAd transduced cells were most likely against the viral proteins (Fig. 2). It was, therefore, important to determine whether synthesis of viral proteins is required de novo in the target cells or whether the incoming viral proteins in the inoculum were sufficient to induce an immune response. To answer this question, we investigated whether RDAd vectors that expressed either LacZ or canine factor IX gene can induce an immune response after being inactivated by UV irradiation in the presence of psoralen. This treatment results in the crosslinking of the viral DNA and consequently prevents transcription, gene expression, and replication from these vectors (37). Our criteria to evaluate the potency of the inactivated RDAd vectors to induce an immune response were (i) their ability to recruit CD4+ and CD8+ cells to the transduced tissue and (ii) the induction of CTL response against major histocompatibility complex-matched target cells that were infected with active or inactivated RDAd. The titer of the inactivated vectors was measured by their ability to induce cytopathic foci on 293 cells (which make E1A protein), which dropped from 1010 units per milliliter before vector inactivation to <102 units per milliliter (the lowest dilution that could be assayed). To have a more quantitative evaluation of the inactivation procedure, we measured the amount of factor IX protein secreted in HeLa cells infected with the inactivated vector at a moi of >10. The amount of canine factor IX secreted into 10 ml of media by 106 infected cells in 48 h was below detection level by ELISA (100 pg/ml) as compared with >2 μg/ml by untreated vector, indicating that the inactivation procedure was very efficient. The inactivated vectors (109 units) were injected into the hind leg muscles of BALB/c mice. Immunostaining of the transduced muscles 5 and 10 days after injection demonstrated extensive infiltration of CD4+ and CD8+ lymphocytes (Fig. 1 I and J). The levels and kinetics of lymphocyte migration were similar to that observed with active RDAd vectors (Fig. 1 A–H). No lymphocyte infiltration was observed when bovine serum albumin was injected in the muscle (Fig. 1 K and L). These data support the notion that de novo synthesis of viral proteins was not a prerequisite for the infiltration of lymphocytes to tissues transduced by RDAd vectors. This observation is in agreement with results showing pulmonary inflammation induced by incomplete or inactivated adenoviral particles (38).
CTLs From Mice Infected with Inactivated LacZ RDAd.
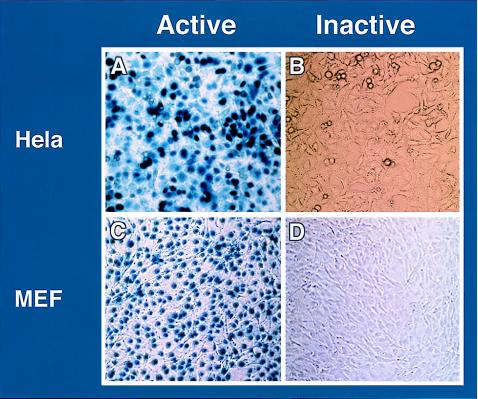
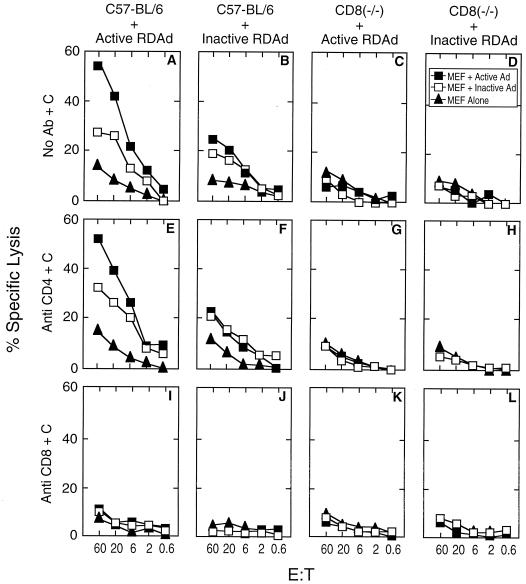
To further substantiate that infection with inactivated RDAd vectors can elicit CTL response, we injected 109 units equivalent of psoralen-UV treated LacZ RDAd into the hind leg muscle of C57/BL6 mice, and splenocytes obtained 3 weeks after injection were used as effector cells in CTL assays. RDAd vector expressing β-galactosidase were inactivated as described above, and when 106 HeLa cells or mouse embryo fibroblasts were infected with inactivated vector at a moi of 50, no β-galactosidase positive cells were observed (Fig. 4 B and D). Thus, there was no detectable transgene expression from the inactivated vector. Mouse embryo fibroblasts infected with active or inactive RDAd vectors were used as target cells. Fig. 5 shows that effector cells from mice infected with either active or inactive LacZ RDAd are able to lyse target cells (Fig. 5 A and B). When CD8(−/−) “knockout” mice (39) were infected with either active or inactive virus, no specific lysis could be detected (Fig. 5 C and D). The CTL assays were specific for CD8+ lymphocytes because overall lysis was not affected by treatment with anti-CD4+ sera (Fig. 5 E–H) whereas no lysis was observed when anti-CD8+ sera was used (Fig. 5 I–L).
Figure 4.
Biological activity of active and inactive RDAd vectors. 109 units of RDAd containing LacZ (active) or psoralen-UV crosslinked (inactive) were infected in HeLa or mouse embryo fibroblasts (MEF). As compared with active vectors (A and C) no β-galactosidase-positive (blue) cells were detected when the vector was inactivated (B and D).
Figure 5.
C57BL6 mice were infected with active Ad β-gal (A, E, and I) or inactive β-gal RDAd (B, F, and J), CD8 −/− knockouts similarly were infected with active (C, G, and K) or inactive virus (D, H, and L). After 3 weeks, cultures were established as described in Materials and Methods. C57BL6 MEF targets were infected as indicated.
It is worth pointing out that the extent of lysis of the target cells is greater when the effector cells were obtained from animals infected with active RDAd. Furthermore, target cells infected with active RDAd were lysed more efficiently than those infected with inactive RDAd (Fig. 5 A and B). Though the overall lysis of target cells was less efficient when effector cells were generated from mice infected with inactive RDAd, there was little difference in the extent of lysis of target cells infected with either active or inactive RDAd vectors (Fig. 5 B and F). Taken together, these results suggest that not all antigenic epitopes present in cells infected with active RDAd are expressed in cells infected with inactive RDAd. We conclude that de novo synthesis of either viral or transgene is not obligatory to induce cellular immune response to cells infected with RDAd vectors.
Induction of CTLs Is Independent of Route of Infection.
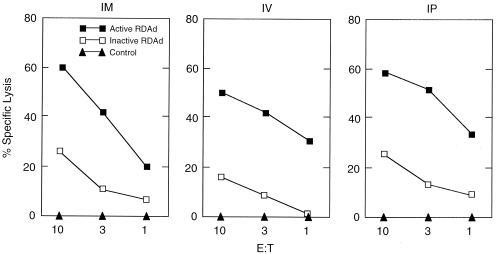
It is possible that efficiency and extent of induction of CTLs is influenced by the site of injection with RDAd vectors. We therefore injected 109 units of β-gal-RDAd i.m., i.v., or i.p. in C57BL6 mice. Splenocytes obtained from infected mice were used as effector cells, and mouse embryo fibroblasts infected with active or inactive β-gal-RDAd were used as target cells. Fig. 6 shows cell lysis in all cases, and, as before, the extent of lysis was higher when the target cells were infected with active virus.
Figure 6.
Routes of infection of RDAd vectors. 109 units of LacZ-RDAd vectors were injected i.m., i.v., or i.p. in C57BL6 mice and effector cells prepared 3 weeks after infection. The target cells were prepared by infecting mouse embryo fibroblasts with 109 units of active virus (■) or inactive virus (□) or were uninfected (▴).
DISCUSSION
Adenoviral vectors fulfill two of the most desirable characteristics of a gene transfer vehicle, namely, (i) the ability to generate high-titer replication deficient recombinant viruses (>1012 virus particles per milliliter), and (ii) efficient gene transfer to a wide variety of dividing and nondividing cells. Unfortunately, expression of the transgene is transient because of the cellular immune response against transduced cells (10, 40). Furthermore, repeated injections with RDAd vectors are hampered severely by the antibodies to the viral proteins. Long term expression from RDAd vectors can be achieved in nude mice or by using immunosuppressive drugs (10). If the immune response to transduced cells can be overcome, adenoviral vectors will have a great potential for in vivo gene delivery.
Role of the Transgene.
The product of the transgene was suspected to be the principle cause of cellular immune response (32). Although it may be true that transgene products can elicit a CTL response, our data shown here with self (murine) or foreign (canine) transgenic proteins (Fig. 1), however, indicate that adenoviral proteins may be a more likely culprit. Both lymphocyte infiltration and CTL lysis observed after infection with RDAd containing either self or foreign transgene was indistinguishable. Recently, it has been shown that infection by gutless adenoviral vectors containing the LacZ gene in LacZ-transgenic mice lead to long term expression, suggesting tolerance in mice producing endogenous β-gal protein (23). Our data, however, are not comparable with these results because (i) the titers of LacZ gutless vectors were 107 units per milliliter (compared with 109 units per milliliter used here) and, more importantly, (ii) neonatal (4-day-old) mice were injected. It has been shown by many investigators (10) that infection of neonatal mice by RDAd vectors does not elicit a cellular immune response.
Role of Viral Proteins in Immune Response.
The first generation of RDAd vectors was not completely replication-defective and, indeed, synthesized late viral mRNA (10). However, the inability of the second and third generation of vectors that were unlikely to synthesize any viral protein to exhibit sustained expression of the transgene prompted us to ask whether the incoming viral proteins in the inoculum may be directly responsible for generating CTLs.
The ability of the psoralen-treated, UV-crosslinked RDAd vectors to generate CTLs lends strong credence to the notion that cellular immunity to RDAd vectors is likely against viral protein(s). Because the inactivated RDAd vectors were biologically inactive as judged by diminished (by >108) formation of cytopathic foci on 293 cells and lack of production of detectable transgene product (Fig. 4), it was a surprise to find extensive CD8+ and CD4+ lymphocyte migration at the site of the injected tissue. Additional evidence of the role of viral proteins in cellular immunity is furnished by CTL assays by using either target cells infected with inactivated RDAd (Fig. 2) or effector cells generated after injection of inactivated RDAd (Fig. 5). Presumably, the inactivated RDAd vectors are released from endosomes (41) and undergo cytosolic processing mechanisms akin to conventional major histocompatibility complex-1 processing and presentation of the peptide to the surface of the infected cell. Inactivated RDAd vectors also may stimulate CTLs by cross-presentation (42–44). It is likely that the machinery used for conventional major histocompatibility complex-1 processing pathway is used by inactivated RDAd vectors, except that the viral proteins are delivered to the cytosol by endocytosis rather than de novo synthesis. It has been shown that cytomegalo virus (CMV)-specific, class I-restricted CTL response in individuals latently infected with CMV is predominantly specific for selected structural viral proteins introduced into the cell after viral penetration and that efficient recognition occurs in the absence of de novo viral synthesis (45).
Gene Therapy.
Recently, long term expression has been achieved with gutless Ad-vectors (31). In these vectors, essentially all of the viral coding domain has been removed and replaced by genes of interest and “stuffer” DNA to make the correct size of adeno DNA for packaging (31). However, the infecting inoculum still contains all of the viral proteins capable of generating CTLs. Yet several groups have shown long term expression in vivo after infection with gutless vectors. This raises the issue as to how some cells infected with gutless Ad-vectors escape immune recognition. It is possible that, although infection by a gutless virus produces CTLs (T.K., unpublished data), some infected cells are not recognized and allow long term production of the transgene. Additionally, although CTLs are generated against specific viral peptides, the effector cells produced may not be able to recognize cells infected by gutless RDAd vectors as target cells. The adeno-specific peptide on the infected cell eventually will disappear because of the normal turnover of class 1 molecules and will escape lysis by CTLs. Thus, these CTLs may be operational only if new viral infections are carried out.
The data presented, here however, have wider implications for adenoviral-based gene therapy approaches. We argue that the incoming viral proteins, at least at titers of 109 units per milliliter, induce and recruit sufficient CTLs, which may eliminate the transduced cell and consequently the loss of the transgene expression. There are, however, other strategies that can bypass this problem: for example, transient subversion of the host immune system by blocking the function of co-stimulatory molecules (19, 23, 40) or the use of immunosuppressive drugs (10, 18). Alternatively, strong tissue specific promoters can be used to transcribe the foreign gene that will reduce the titers of RDAd vectors required for generating therapeutic amounts of the transgenic product. The currently available RDAd vectors remain extremely useful for introducing foreign genes in which only transient expression is required: for instance, cancer therapy and blocking or augmenting expression during development and differentiation. For sustained production of the foreign protein or readministration of the foreign gene, the adenoviral vectors still face a formidable challenge.
Acknowledgments
We are grateful to Drs. M. Zoppe, Y. Kanagae, and I. Saito for providing some adenoviral vectors, Wai Ping Fong Leung for providing CD8(−/−) knockout mice, and Ling Ouyang for technical assistance. We thank Drs. D. Pardol, P. Greenberg, and H. Ploegh for critical reading of the manuscript; Drs. J. Leiden, G. Nabel, and J. Wilson for discussions. Tal Kafri is supported by a postdoctoral fellowship from The Cystic Fibrosis Foundation. T. Krahl and N. Sarvetnik gratefully acknowledge the Diabetes Intradisciplinary Center. This work was supported by grants from the March of Dimes Foundation, the Francis Berger Foundation and the National Institutes of Health (Grant CA57855 to L.S. and Grant OIG to I.V.). Dr. Verma is an American Cancer Society Professor of Molecular Biology.
ABBREVIATIONS
- RDAd
replication-defective adenoviral
- CTL
cytotoxic T lymphocyte
- moi
multiplicity of infection
- CMV
cytomegalo virus
References
- 1.Verma I M, Somia N. Nature (London) 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 2.Anderson W F. Nature (London) 1998;392:25–30. doi: 10.1038/32058. [DOI] [PubMed] [Google Scholar]
- 3.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 4.Blomer U, Naldini L, Kafri T, Trono D, Verma I M, Gage F H. J Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kafri T, Blomer U, Peterson D A, Gage F H, Verma I M. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 6.Kremer E J, Perricaudet M. Br Med Bull. 1995;51:31–44. doi: 10.1093/oxfordjournals.bmb.a072951. [DOI] [PubMed] [Google Scholar]
- 7.Kozarsky K F, Wilson J M. Curr Opin Genet Dev. 1993;3:499–503. doi: 10.1016/0959-437x(93)90126-a. [DOI] [PubMed] [Google Scholar]
- 8.Brody S L, Crystal R G. Ann NY Acad Sci. 1994;716:90–103. doi: 10.1111/j.1749-6632.1994.tb21705.x. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Nunes F A, Berencsi K, Furth E E, Gonczol E, Wilson J M. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai Y, Schwarz E M, Gu D, Zhang W W, Sarvetnick N, Verma I M. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Greenough K, Wilson J M. Gene Ther. 1996;3:412–420. [PubMed] [Google Scholar]
- 12.Yang Y, Li Q, Ertl H C, Wilson J M. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Ertl H C, Wilson J M. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 14.Kay M A, Landen C N, Rothenberg S R, Taylor L A, Leland F, Wiehle S, Fang B, Bellinger D, Finegold M, Thompson A R, et al. Proc Natl Acad Sci USA. 1994;91:2353–2357. doi: 10.1073/pnas.91.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tripathy S K, Goldwasser E, Lu M M, Barr E, Leiden J M. Proc Natl Acad Sci USA. 1994;91:11557–11561. doi: 10.1073/pnas.91.24.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichel R, Kovesdi I, Nevins J R. Proc Natl Acad Sci USA. 1988;85:387–390. doi: 10.1073/pnas.85.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shenk T, Williams J. CurrTop Microbiol Immunol. 1984;111:1–39. doi: 10.1007/978-3-642-69549-0_1. [DOI] [PubMed] [Google Scholar]
- 18.Engelhardt J F, Ye X, Doranz B, Wilson J M. Proc Natl Acad Sci USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kay M A, Holterman A X, Meuse L, Gown A, Ochs H D, Linsley P S, Wilson C B. Nat Genet. 1995;11:191–197. doi: 10.1038/ng1095-191. [DOI] [PubMed] [Google Scholar]
- 20.Mitani K, Graham F L, Caskey C T, Kochanek S. Proc Natl Acad Sci USA. 1995;92:3854–3858. doi: 10.1073/pnas.92.9.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochanek S, Clemens P R, Mitani K, Chen H H, Chan S, Caskey C T. Proc Natl Acad Sci USA. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clemens P R, Kochanek S, Sunada Y, Chan S, Chen H H, Campbell K P, Caskey C T. Gene Ther. 1996;3:965–972. [PubMed] [Google Scholar]
- 23.Chen H H, Mack L M, Kelly R, Ontell M, Kochanek S, Clemens P R. Proc Natl Acad Sci USA. 1997;94:1645–1650. doi: 10.1073/pnas.94.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 25.Kumar-Singh R, Chamberlain J S. Hum Mol Genet. 1996;5:913–921. doi: 10.1093/hmg/5.7.913. [DOI] [PubMed] [Google Scholar]
- 26.Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps M L. J Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lieber A, He C Y, Kirillova I, Kay M A. J Virol. 1996;70:8944–8960. doi: 10.1128/jvi.70.12.8944-8960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parks R J, Chen L, Anton M, Sankar U, Rudnicki M A, Graham F L. Proc Natl Acad Sci USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haecker S E, Stedman H H, Balice-Gordon R J, Smith D B, Greelish J P, Mitchell M A, Wells A, Sweeney H L, Wilson J M. Hum Gene Ther. 1996;7:1907–1914. doi: 10.1089/hum.1996.7.15-1907. [DOI] [PubMed] [Google Scholar]
- 30.Schiedner G, Morral N, Parks R J, Wu Y, Koopmans S C, Langston C, Graham F L, Beaudet A L, Kochanek S. Nat Genet. 1998;18:180–183. doi: 10.1038/ng0298-180. [DOI] [PubMed] [Google Scholar]
- 31.Morsy M A, Gu M, Motzel S, Zhao J, Lin J, Su Q, Allen H, Franlin L, Parks J R, Kochanek S, et al. Proc Natl Acad Sci USA. 1998;95:7866–7871. doi: 10.1073/pnas.95.14.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tripathy S K, Black H B, Goldwasser E, Leiden J M. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 34.Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K, Tokuda C, Saito I. Proc Natl Acad Sci USA. 1996;93:1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanegae Y, Lee G, Sato Y, Tanaka M, Nakai M, Sakaki T, Sugano S, Saito I. Nucleic Acids Res. 1995;23:3816–3821. doi: 10.1093/nar/23.19.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Zoppe M, Hackeng T M, Griffin J H, Lee K F, Verma I M. Proc Natl Acad Sci USA. 1997;94:11563–11566. doi: 10.1073/pnas.94.21.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cotten M, Saltik M, Kursa M, Wagner E, Maass G, Birnstiel M L. Virology. 1994;205:254–261. doi: 10.1006/viro.1994.1641. [DOI] [PubMed] [Google Scholar]
- 38.McCoy R D, Davidson B L, Roessler B J, Huffnagle G B, Janich S L, Laing T J, Simon R H. Hum Gene Ther. 1995;6:1553–1560. doi: 10.1089/hum.1995.6.12-1553. [DOI] [PubMed] [Google Scholar]
- 39.Fung-Leung W P, Schilham M W, Rahemtulla A, Kundig T M, Vollenweider M, Potter J, van Ewijk W, Mak T W. Cell. 1991;65:443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Su Q, Wilson J M. J Virol. 1996;70:7209–7212. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greber U F, Willetts M, Webster P, Helenius A. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 42.Bevan M J. J Exp Med. 1995;182:639–641. doi: 10.1084/jem.182.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harding C V. J Clin Immunol. 1996;16:90–96. doi: 10.1007/BF01540955. [DOI] [PubMed] [Google Scholar]
- 44.Rock K L. Immunol Today. 1996;17:131–137. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 45.Riddell S R, Rabin M, Geballe A P, Britt W J, Greenberg P D. J Immunol. 1991;146:2795–2804. [PubMed] [Google Scholar]