Abstract
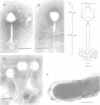
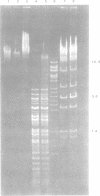
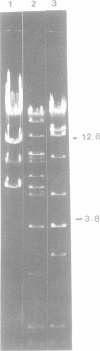
The first generalized transducing bacteriophage reported for Bacillus megaterium has been characterized. Optimum conditions for lysate production and transduction procedures were established so that transducing frequencies of 8 x 10(-6) and higher are now possible. The phage, MP13, has a head diameter of 97 nm and a contractile tail (202 by 17 nm) and adsorbs to the periphery of the cell. MP13 was inactivated rapidly at 60 degrees C, but not at 55 degrees C, and was sensitive to toluene, ether, and chloroform. When centrifuged in a neutral CsCl gradient, two bands were observed, a major band of 1.490 g cm-3 and a minor band of 1.482 g cm-3 buoyant density. The major band contained only infective particles, whereas the minor band contained both infective and transducing particles. Phage DNA was resistant to several restriction endonucleases, but yielded 9 fragments with MboI, more than 34 with HindIII, and 7 with BstEII. The molecular weights for the fragments from MboI-BstEII double digests total 97 x 10(9).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biswal N., Kleinschmidt A. K., Spatz H. C., Trautner T. A. Physical properties of the DNA of bacteriophage SP50. Mol Gen Genet. 1967;100(1):39–55. doi: 10.1007/BF00425774. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. J., Carlton B. C. Plasmid-mediated transformation in Bacillus megaterium. J Bacteriol. 1980 May;142(2):508–512. doi: 10.1128/jb.142.2.508-512.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho P. M., Vary J. C. Isolation and characterization of a Bacillus megaterium QMB1551 bacteriophage. J Gen Virol. 1977 Sep;36(3):547–550. doi: 10.1099/0022-1317-36-3-547. [DOI] [PubMed] [Google Scholar]
- Clark R. W., Wever G. H., Wiberg J. S. High-molecular-weight DNA and the sedimentation coefficient: a new perspective based on DNA from T7 bacteriophage and two novel forms of T4 bacteriophage. J Virol. 1980 Jan;33(1):438–448. doi: 10.1128/jvi.33.1.438-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney P. H., Jacob R. J., Slepecky R. A. Characteristics of a Bacillus megaterium bacteriophage. J Gen Virol. 1975 Jan;26(1):131–134. doi: 10.1099/0022-1317-26-1-131. [DOI] [PubMed] [Google Scholar]
- Decker S. J., Lang D. R. Bacillus megaterium mutant deficient in membrane-bound adenosine triphosphatase activity. J Bacteriol. 1977 Jul;131(1):98–104. doi: 10.1128/jb.131.1.98-104.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor K., Alföldi L. Polyethylene-glycol induced fusion of bacterial protoplasts: direct selection of recombinants. Mol Gen Genet. 1979 Jan 5;168(1):55–59. doi: 10.1007/BF00267933. [DOI] [PubMed] [Google Scholar]
- Frampton E. W., Mandel M. Properties of the deoxyribonucleic acid contained in the defective particle coliphage 15. J Virol. 1970 Jan;5(1):8–13. doi: 10.1128/jvi.5.1.8-13.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry G. S., Fitz-James P. C. Characteristics of phi T, the temperate bacteriophage carried by Bacillus megaterium 899a. J Virol. 1974 Feb;13(2):494–499. doi: 10.1128/jvi.13.2.494-499.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Trautner T. A. Maturation of bacteriophage SPPI DNA: limited precision in the sizing of mature bacteriophage genomes. J Virol. 1981 Feb;37(2):832–835. doi: 10.1128/jvi.37.2.832-835.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. An accurate measurement of the catalase crystal period and its use as an internal marker for electron microscopy. J Ultrastruct Res. 1967 Sep;20(1):91–102. doi: 10.1016/s0022-5320(67)80038-8. [DOI] [PubMed] [Google Scholar]
- Marmur J., Brandon C., Neubort S., Ehrlich M., Mandel M., Konvicka J. Unique properties of nucleic acid from Bacillus subtilis phage SP-15. Nat New Biol. 1972 Sep 20;239(90):68–70. doi: 10.1038/newbio239068a0. [DOI] [PubMed] [Google Scholar]
- Neubort S., Marmur J. Synthesis of the unusual DNA of Bacillus subtilis bacteriophage SP-15. J Virol. 1973 Nov;12(5):1078–1084. doi: 10.1128/jvi.12.5.1078-1084.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postemsky C. J., Dignam S. S., Setlow P. Isolation and characterization of Bacillus megaterium mutants containing decreased levels of spore protease. J Bacteriol. 1978 Sep;135(3):841–850. doi: 10.1128/jb.135.3.841-850.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay L. K., Vary J. C. Biochemical studies on glucose initiated germination in Bacillus megaterium. Biochim Biophys Acta. 1978 Jan 18;538(2):284–292. doi: 10.1016/0304-4165(78)90356-2. [DOI] [PubMed] [Google Scholar]
- Taylor M. J., Thorne C. B. Concurrent changes in transducing efficiency and content of transforming deoxyribonucleic acid in Bacillus subtilis bacteriophage SP-10. J Bacteriol. 1966 Jan;91(1):81–88. doi: 10.1128/jb.91.1.81-88.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne C. B., Stull H. B. Factors affecting transformation of Bacillus licheniformis. J Bacteriol. 1966 Mar;91(3):1012–1020. doi: 10.1128/jb.91.3.1012-1020.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne C. B. Transduction in Bacillus thuringiensis. Appl Environ Microbiol. 1978 Jun;35(6):1109–1115. doi: 10.1128/aem.35.6.1109-1115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyeryar F. J., Jr, Taylor M. J., Lawton W. D., Goldberg I. D. Cotransduction and cotransformation of genetic markers in Bacillus subtilis and Bacillus licheniformis. J Bacteriol. 1969 Nov;100(2):1027–1036. doi: 10.1128/jb.100.2.1027-1036.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary J. C., Kornberg A. Biochemical studies of bacterial sporulation and germination. XXI. Temperature-sensitive mutants for initiation of germination. J Bacteriol. 1970 Jan;101(1):327–329. doi: 10.1128/jb.101.1.327-329.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary J. C. Properties of Bacillus megaterium temperature-sensitive germination mutants. J Bacteriol. 1975 Jan;121(1):197–203. doi: 10.1128/jb.121.1.197-203.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary J. C. Spore germination of Bacillus megaterium QM B1551 mutants. J Bacteriol. 1972 Oct;112(1):640–642. doi: 10.1128/jb.112.1.640-642.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary P. S., Halsey W. F. Host-range and partial characterization of several new bacteriophages for Bacillus megaterium QM b1551. J Gen Virol. 1980 Nov;51(Pt 1):137–146. doi: 10.1099/0022-1317-51-1-137. [DOI] [PubMed] [Google Scholar]
- Vary P. S. Transduction in Bacillus megaterium. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1119–1124. doi: 10.1016/0006-291x(79)91524-9. [DOI] [PubMed] [Google Scholar]
- Wachsman J. T., Hogg L. Use of thymineless death to enrich for doubly auxotrophic mutants of Bacillus megaterium. J Bacteriol. 1964 May;87(5):1118–1122. doi: 10.1128/jb.87.5.1118-1122.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax R., Freese E., Cashel M. Separation of two functional roles of L-alanine in the initiation of Bacillus subtilis spore germination. J Bacteriol. 1967 Sep;94(3):522–529. doi: 10.1128/jb.94.3.522-529.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehle C. O., Doi R. H. Differential expression of bacteriophage genomes in vegetative and sporulating cells of Bacillus subtilis. J Virol. 1967 Oct;1(5):935–947. doi: 10.1128/jvi.1.5.935-947.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton D. B., Thorne C. B. Transduction in Bacillus cereus by each of two bacteriophages. J Bacteriol. 1970 May;102(2):573–579. doi: 10.1128/jb.102.2.573-579.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]