Abstract
Discovered in the early 1960s as a T cell cytokine, the protein mediator known as macrophage migration inhibitory factor (MIF) has been found recently to be a pituitary peptide released during the physiological stress response, a proinflammatory macrophage cytokine secreted after LPS stimulation, and a T cell product expressed as part of the antigen-dependent activation response. We report herein that MIF also plays a critical role in the innate host response to staphylococcal and streptococcal exotoxins. In RAW 264.7 or elicited mouse peritoneal macrophages, peak MIF secretion was induced by concentrations of the staphylococcal toxic shock syndrome (TSS) toxin 1 (TSST-1) and the streptococcal pyrogenic exotoxin A as low as 10 pg/ml. Moreover, dose-response studies of splenocyte cytokine production showed that lower concentrations of TSST-1 (10 pg/ml) were needed to release MIF than to induce interleukin 2 or interferon-γ secretion (1 ng/ml). We also studied the effect of neutralizing anti-MIF antibodies on TSST-1-induced lymphocyte proliferation and lethal toxic shock. Pretreatment of C57BL/6 mice with anti-MIF antibody 2 hr before TSST-1 injection prevented spleen enlargement and reduced by 50% the proliferation of splenocytes measured ex vivo. In a lethal mouse model of TSST-1-induced shock, anti-MIF antibody increased survival from 8% to 54% (P < 0.0001). These studies indicate that Gram-positive exotoxins are extremely potent inducers of MIF secretion and establish a critical role for MIF and the macrophage in the pathogenesis of the TSSs and in the innate immune response.
Keywords: T lymphocyte/cytokine/toxic shock syndrome toxin 1/superantigens/septic shock
The biochemical nature and biological function of the protein known as macrophage migration inhibitory factor (MIF), which was described originally to be a T lymphocyte product that inhibited macrophage migration, remained enigmatic for almost three decades (1–4). Recent findings, however, have ascribed a pivotal role to MIF within the immune and endocrine systems. First, the sequence structure of a newly identified protein that was released by pituitary cells in a hormone-like fashion was found to be that of MIF (5–7). Second, monocytes and macrophages that had previously been considered to be the target of MIF action, were observed to be a significant source of MIF after stimulation with endotoxin (lipopolysaccharide, or LPS) or with the cytokines tumor necrosis factor α (TNFα) and interferon-γ (IFNγ) (8). MIF also was described to mediate certain pro-inflammatory effects, stimulating macrophages to produce TNFα and nitric oxide when given in combination with IFNγ (8, 9). Like TNFα and IL-1β, MIF plays a central role in the host response to endotoxemia. Coinjection of recombinant MIF and LPS exacerbates LPS lethality, whereas neutralizing anti-MIF antibodies fully protect mice from endotoxic shock. Similar observations have been made recently in a mouse model of Escherichia coli peritonitis (T.C., unpublished observations). Studies of MIF expression by mouse and human T lymphocytes also established that MIF is a proinflammatory T cell cytokine that is required for T cell activation and antibody production by B cells (10). Finally, the critical regulatory role played by MIF was underscored by the finding that glucocorticoids at low dose stimulated the production of MIF by macrophages and T cells, the first such response ascribed to glucocorticoids to date (6, 10). Importantly, MIF has been shown to function to control or “counter-balance” the anti-inflammatory and immunosuppressive effects of glucocorticoids on macrophages and T cells (6, 10, 11).
The proportion of severe infections and septic shock caused by Gram-positive bacteria has increased markedly in recent years, such that these pathogens now account for 40–50 percent of all cases of septic shock occurring in the intensive care setting (12). Staphylococcal and streptococcal toxic-shock syndromes (TSS) and viridans streptococcal infections accompanied by shock or the adult respiratory distress syndrome are examples of the fulminant and often fatal complications of Gram-positive sepsis. In contrast to Gram-negative septic shock, very little is known about the pathophysiology of Gram-positive infections leading to septic shock. In the case of TSS for instance, staphylococcal and streptococcal exotoxins appear to cause a massive activation of macrophages and T lymphocytes, which leads to the production of high levels of proinflammatory cytokines (13–18). Many Gram-positive bacteria do not produce exotoxins however, and they cause shock by mechanisms that remain to be fully unraveled.
Given the central regulatory role of MIF in both the macrophage and the T cell limbs of the acute inflammatory and immune responses, we have investigated the extent as well as the role of MIF expression in the host response to Gram-positive exotoxins. In this study, we report that the TSS toxin-1 (TSST-1) and the streptococcal pyrogenic exotoxin A (SPEA) are very potent inducers of MIF production by immune cells and that MIF is an important mediator of lymphocyte activation and toxic shock triggered by these toxins.
MATERIALS AND METHODS
Reagents.
TSST-1 and streptococcal pyrogenic exotoxin A (SPEA) were obtained from Toxin Technology (Sarasota, FL). According to the manufacturer, the toxins were ≥95% pure and the LPS content of all the batches used ranged between 0.02–0.075 endotoxin unit (equal to 2–7.5 pg of LPS) per μg of proteins. TSST-1 did not react with antibodies to the staphylococcal enterotoxins A, B, C, D, and E or to the exfoliative toxin A. SPEA did not react with antibodies to the streptococcal pyrogenic exotoxins B and C. The toxins were resuspended in pyrogen-free water at a concentration of 1 mg/ml, aliquoted and stored at −80°C. Anti-IL-2 mAb was from Genzyme. d-Galactosamine, carbenicillin, Tween-20 were obtained from Sigma. Gentamicin was from GIBCO. Thioglycollate broth (Difco) was prepared according to the manufacturer’s recommendation, autoclaved, and stored protected from light at room temperature. Horseradish peroxidase-conjugated goat anti-rabbit antibody was purchased from Pierce and 4-chloro-1-naphthol and stabilized 3,3′,5,5′-tetramethylbenzidene substrate for horseradish peroxidase were from Promega. Polyclonal anti-MIF serum was generated by immunizing New Zealand White rabbits (Hare Marland, Hewitt, NJ) with purified, mouse recombinant MIF as described (8). Anti-MIF and nonimmune IgG were isolated from serum by protein-G affinity chromatography following the manufacturer’s recommendations (Pharmacia).
Cells.
Mouse RAW 264.7 macrophages and AtT-20 anterior pituitary cells were from the American Type Culture Collection (Manassas, VA). Thioglycollate-elicited peritoneal macrophages were obtained from BALB/c mice that were injected i.p. with 2 ml of sterile thioglycollate broth. Seventy-two hours after injection, cells were harvested by lavage of the peritoneal cavity with 5 ml of an ice-chilled 11.6% sucrose solution under aseptic conditions. Spleen cells (splenocytes) suspensions obtained from three to six BALB/c or C57BL/6 mice were pooled, and the red blood cells lysed by treatment with 0.8% NH4Cl (19). After washing and centrifugation (10 min at 800 × g), cells were resuspended in medium, enumerated and plated. Cells were cultured in RPMI 1640 medium (GIBCO) containing 2 mM glutamine, 50 μg/ml of carbenicillin and gentamicin, and 10% heat-inactivated fetal bovine serum (HyClone) or 1% homologous mouse serum (splenocytes).
MIF Production by Macrophages, Pituitary Cells and Splenocytes Stimulated with TSST-1 or SPEA.
RAW 264.7 or thioglycollate-elicited peritoneal macrophages or AtT-20 anterior pituitary cells were incubated at 2–3 × 106 cells per well in 3.5-cm tissue culture plates (Linbro, Flow Laboratories). After 3 hr of incubation at 37°C in a humidified atmosphere with 5% CO2, nonadherent cells were removed and wells were washed twice with RPMI/1% fetal bovine serum. Cells then were incubated for 12–14 hr with TSST-1 or SPEA at concentrations ranging from 1 pg/ml to 100 ng/ml. For time-course experiments, conditioned media of parallel cultures were removed at 0.5, 1, 2, 4, and 8 hr intervals after stimulation with 1 ng/ml (RAW 264.7 cells) or 10 pg/ml (AtT-20 cells) of TSST-1. The MIF content of the cell-conditioned media was analyzed by Western blotting and by ELISA. Splenocytes (5 × 105 cells) were cultured in 48-well tissue culture plates (Costar) in RPMI containing 1% of homologous mouse serum and incubated with TSST-1 at 0.01, 1, 10, 100, and 1,000 ng/ml. Sixty microliters of cell-conditioned media was removed after 24 hr to determine the concentrations of MIF and IL-2, and after 72 hr to determine the concentration of IFNγ. Cytokine concentrations were determined by ELISA. The IL-2 and IFNγ ELISA kits were from Endogen (Cambridge, MA).
Western Blotting and ELISA for Detection of MIF.
For Western blotting analyses, cell-conditioned media were centrifuged (10 min at 800 g) and the supernatants concentrated 10-fold by membrane filtration (10 kDa cut-off) (Centricon-10, Amicon). Samples then were resolved on SDS/18% polyacrylamide gels under reducing conditions and transferred onto nitrocellulose membrane (Schleicher & Schuell) at 50 V and 150 mA for 16 hr. Membranes were processed as previously described (8). MIF concentrations in cell-conditioned media were quantified by a sandwich ELISA using a monoclonal anti-mouse MIF capture antibody, a polyclonal rabbit anti-mouse MIF detector, and purified mouse recombinant MIF as standard (6, 11). Samples were analyzed in triplicate and the results expressed as mean ± SEM of two or three separate experiments.
Effect of Anti-MIF Antibody on Splenocyte Proliferation in Vitro.
BALB/c splenocytes (4 × 105 cells) were cultured in 96-well flat bottom tissue culture plates (Linbro) in RPMI containing 1% homologous mouse serum. Cells were incubated for 72 hr with TSST-1 at 0.01, 1, 10, and 100 ng/ml in the presence of nonimmune IgG, anti-MIF IgG (each at 50 μg/ml) or anti-IL-2 IgG (5 μg/ml). Cells then were pulsed for 6 hr with [3H]thymidine (1 μCi per well; 1 Ci = 37 GBq), harvested and the incorporation of [3H]thymidine into DNA measured by liquid scintillation counting in a microplate reader (TopCount, Packard).
Effect of Anti-MIF Antibody on Splenocyte Proliferation in Vivo.
Eight to 10 week-old (19–21 g) female C57BL/6 mice (Charles River Breeding Laboratories) were housed in groups of 5 or 10 mice per cage with free access to food and water and were acclimatized for at least 5 days before experimentation. Groups of three mice were injected i.p. with 200 μl of anti-MIF or nonimmune rabbit serum 2 hr before i.p. injection of TSST-1 (average dose per mouse: 20 μg, i.e., 1 mg/kg body weight) diluted into 0.2 ml of saline. After 72 hr, mice were euthanized by CO2 asphyxiation and the spleens removed and weighed. Spleen cells suspensions were prepared as described above, pooled, washed, resuspended in RPMI/1% homologous mouse serum and plated at a density of 5 × 105 cells per well in 96-well plates. Cells then were pulsed for 6hr with [3H]thymidine (0.5 μCi per well), harvested and the incorporation of [3H]thymidine into DNA measured by liquid scintillation counting.
Murine Model of TSS.
Eight to 10 week-old (19–21 g) female BALB/c mice (Charles River Labs, Kingston, NY) were housed and acclimatized as described above. On the day of the experiment, mice were weighed and randomly distributed into groups of 8 to 10 animals of equal mean body weight. Mice were injected i.p. with 200 μl of anti-MIF or nonimmune rabbit serum 2 hr before the i.p. injection of TSST-1 (average dose per mouse 20 μg, i.e., 1 mg/kg body weight) and d-galactosamine (18 mg per mouse, i.e., 0.9 g/kg body weight) diluted into 0.25 ml of saline. Mice were observed for at least seven days.
RESULTS
MIF Production by Macrophages Stimulated with TSST-1 or SPEA.
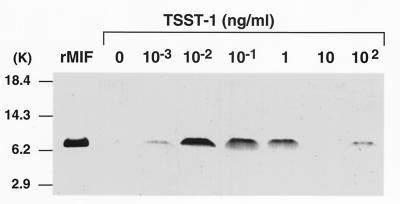
Previous observations that the macrophage is an abundant source of MIF that is released after stimulation with LPS (8) led us to examine the secretion of MIF by murine macrophages after activation with exotoxins of Gram-positive bacteria. As shown in Fig. 1, macrophage MIF was found to be released into cell-conditioned media after stimulation with TSST-1. A bell-shaped MIF secretion response was consistently observed. In RAW 264.7 macrophages, MIF release peaked at 10 pg/ml of TSST-1 and decreased at high concentrations of TSST-1, an effect seen previously after stimulation with LPS or dexamethasone (6, 8). As shown in Table 1, a very similar MIF secretory profile, but of lower magnitude, was observed when primary, thioglycollate-elicited mouse peritoneal macrophages were stimulated with TSST-1. The time-course of MIF secretion then was examined in parallel cultures of elicited peritoneal macrophages stimulated with 1 ng/ml of TSST-1 for 0.5, 1, 2, 4 and 8 hr. MIF was already detectable in the cell-conditioned media 30–60 min after addition of TSST-1 (450 pg/ml), but the highest levels were measured between 1 and 4 hr post-stimulation (973 pg/ml) (data not shown).
Figure 1.
TSST-1-induced MIF production by mouse macrophages. RAW 264.7 macrophages (3 × 106 cells) were stimulated for 12 hr with TSST-1 at the indicated concentrations. The content of MIF secreted into the medium was analyzed by Western blotting as described in Materials and Methods. Mouse recombinant MIF (10 ng) was electrophoresed and transferred as a standard. MIF concentrations in cell-conditioned media also were quantified by ELISA. Samples were analyzed in triplicate and results expressed as mean ± SEM of three separate experiments. MIF concentrations were 204 ± 149 pg/ml (control cells), and 396 ± 307 pg/ml, 1,694 ± 467 pg/ml, 1,156 ± 380 pg/ml, 1,009 ± 61 pg/ml, 410 ± 282 pg/ml and 321 ± 297 pg/ml in supernatants of cells induced with 10−3, 10−2, 10−1, 1, 10, and 102 ng/ml of TSST-1, respectively.
Table 1.
MIF production by mouse peritoneal macrophages stimulated by TSST-1
| Concentration of TSST-1, ng/ml | Macrophage MIF production, pg/ml
|
|
|---|---|---|
| Mean | SEM | |
| 0 | 130 | 130 |
| 0.001 | 135 | 135 |
| 0.01 | 626 | 360 |
| 0.1 | 259 | 7 |
| 1 | 340 | 74 |
| 10 | 215 | 215 |
| 100 | 146 | 146 |
Thioglycollate-elicited peritoneal macrophages (2 × 106 cells) were incubated for 12–14 hr with TSST-1 at the indicated concentrations and the MIF content of the cell-conditioned media was analyzed by ELISA. Samples were analyzed in triplicate and results expressed as mean ± SEM of two separate experiments.
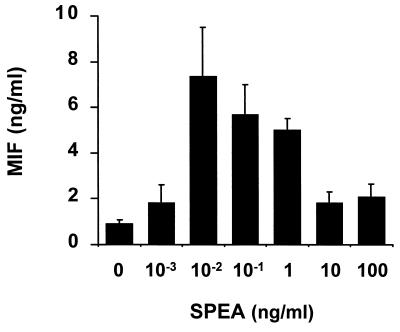
To investigate the effect of another Gram-positive exotoxin on macrophage MIF secretion, we also stimulated RAW 264.7 cells with SPEA. Like TSST-1, SPEA was found to be a strong inducer of macrophage MIF production (Fig. 2). As little as 1 pg/ml of SPEA was sufficient to induce MIF secretion, which peaked at 10 pg/ml and decreased at higher SPEA concentrations. Two Gram-positive exotoxins from Staphylococcus aureus (TSST-1) or from Streptococcus pyogenes (SPEA) thus were found to be very potent inducers of MIF secretion from mouse macrophages.
Figure 2.
SPEA-induced MIF production by mouse macrophages. RAW 264.7 macrophages (2 × 106 cells) were stimulated for 12 hr with SPEA at the indicated concentrations. The MIF content of the cell-conditioned media was analyzed by ELISA. Data are expressed as the mean ± SEM of three separate experiments.
MIF Production by Pituitary Cells Stimulated with TSST-1.
Recent investigations have revealed that MIF is a cytokine produced by the pituitary gland after LPS stimulation (5). We therefore examined whether the Gram-positive exotoxin TSST-1 stimulated MIF release from AtT-20 anterior pituitary cells, an established line of mouse corticotrophic cells. TSST-1 was found to be a powerful inducer of MIF secretion by AtT-20 cells. Maximum production of MIF was triggered by 0.01 pg/ml of TSST-1. Time-course analyses of MIF appearance in cell-conditioned media indicated that 10 pg/ml of TSST-1 induced a rapid release of preformed MIF protein from AtT-20 cells, that peaked at 30 min (MIF concentrations measured by ELISA were 3,300 pg/ml, 2,976 pg/ml, 1,679 pg/ml, and 1,107 pg/ml at 30, 60, 120, and 240 min. poststimulation). Thus, secretion of pituitary MIF was induced by minute quantities of a staphylococcal exotoxin.
Comparison of MIF, IL-2, and IFNγ Production by Mouse Splenocytes Stimulated with TSST-1.
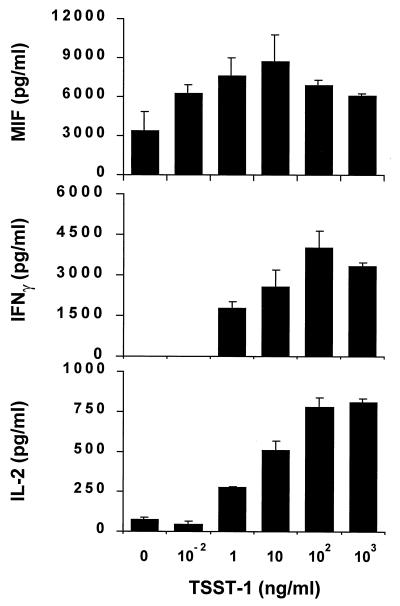
TSST-1 has been shown previously to induce the secretion of TNFα, lymphotoxin, IL-1β, IL-2, and IFNγ from macrophages or T lymphocytes (13–16, 20, 21). The fact that TSST-1 induces MIF secretion from macrophages and that MIF is a proinflammatory T cell cytokine led us to study MIF secretion by murine splenocytes stimulated with TSST-1 and to compare it with that of IL-2 and IFNγ. Splenocytes from BALB/c mice were stimulated with concentrations of TSST-1 ranging from 0.01 to 1,000 ng/ml and the cell-conditioned media harvested after 24 hr for measurements of MIF and IL-2 and after 72 hr for measurement of IFNγ. As shown in Fig. 3, TSST-1 induced the secretion of abundant quantities of MIF by mouse splenocytes. The splenocyte MIF response to increasing concentrations of TSST-1 followed a bell-shaped curve, a finding that is in agreement with data obtained in macrophages (Fig. 1 and Table 1). The amount of TSST-1 needed to induce half-maximum cytokine release was ≈10 pg/ml for MIF and 5 ng/ml for IL-2 or for IFNγ. These data indicate that TSST-1 is a potent inducer of MIF secretion by splenocytes and that the threshold for MIF release after TSST-1 stimulation is inferior to that of the classical T cell cytokines IL-2 and IFNγ.
Figure 3.
Concentrations of MIF, IL-2 and IFNγ in cell-culture supernatants of mouse splenocytes stimulated with TSST-1. Splenocytes (4 × 105) were obtained from BALB/c mice as described in Materials and Methods and stimulated with TSST-1 at the indicated concentrations. Cell-conditioned media was sampled after 24 hr for measurement of MIF and IL-2, and after 72 hr for measurement of IFNγ. Cytokine concentrations were determined by ELISA. Data are expressed as the mean ± SEM of three separate experiments. Levels of IFNγ were undetectable at TSST-1 concentrations below 1 ng/ml.
MIF Is a Mediator of Lymphocyte Activation induced by TSST-1.
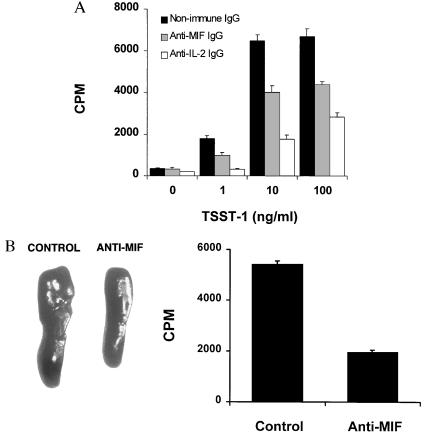
TSST-1 is a powerful trigger of T cell activation that acts by engaging both major histocompatibility complex class II and specific Vβ subunits of the T cell receptor (22). Because MIF was recently shown to play an important regulatory role in T cell activation after mitogenic or antigenic stimuli (10), we examined whether MIF was involved in the proliferative response of splenic lymphocytes by TSST-1. Splenocytes from BALB/c mice were stimulated in vitro with TSST-1 in the presence of neutralizing anti-MIF IgG, anti-IL-2 IgG, or nonimmune (control) IgG. Anti-MIF antibody reduced by 40% the proliferation of splenocytes induced with TSST-1 (Fig. 4A). Used as a positive control, anti-IL-2 antibody inhibited proliferation by 70%.
Figure 4.
Anti-MIF antibody inhibits TSST-1-induced proliferation of mouse splenocytes. (A) Effect of anti-MIF antibody on the proliferation of splenocytes stimulated by TSST-1 in vitro. Splenocytes (4 × 105 cells) from BALB/c mice were stimulated for 72 hr with TSST-1 at the indicated concentrations in the presence of neutralizing anti-MIF IgG (50 μg/ml)(stippled columns), nonimmune IgG (50 μg/ml) (solid columns), or anti-IL-2 IgG (5 μg/ml) (open columns). Cells then were pulsed with [3H]thymidine (0.5 μCi/well) for 6 hr and the incorporation of thymidine measured by liquid scintillation counting. Data from one representative experiment are shown and expressed as the mean ± SEM of quintuplicate values. (B). Effect of anti-MIF antibody on the proliferation of splenocytes stimulated by TSST-1 in vivo. C57BL/6 mice were injected i.p. with 200 μl of anti-MIF or nonimmune (control) rabbit serum 2 hr before 20 μg of TSST-1. After 3 days, spleens were harvested and weighed. Spleens of two representative mice treated with nonimmune serum or anti-MIF serum are shown in the left panel. Splenocytes (5 × 105 cells per well) were prepared as described in Materials and Methods, pulsed for 6 hr with [3H]thymidine (0.5 μCi/well) and the incorporation of thymidine measured by liquid scintillation counting. Data from three separate experiments (n = 8 mice per treatment group) are expressed as the mean cpm ± SD of splenocyte cultures tested in quintuplicate (Right).
To further explore the role of MIF in lymphocyte activation in vivo, we administered neutralizing anti-MIF serum or nonimmune (control) serum to C57BL/6 mice 2 hr before the i.p. injection of TSST-1. Spleens were harvested three days after challenge, weighed, and the splenocytes pulsed with [3H]thymidine for 6 hr. As shown in Fig. 4B, anti-MIF antibody minimized spleen enlargement and markedly reduced lymphocyte activation as measured by [3H]thymidine incorporation ex vivo. The mean ± SD spleen weight of eight mice injected with anti-MIF serum was 166 ± 6.7 mg as compared with 208 ± 4.5 mg for mice injected with nonimmune serum (P = 0.002, two-tailed Student t test). Anti-MIF antibody inhibited [3H]thymidine incorporation of splenocytes by 64% (Fig. 4B Right). Thus, neutralization of the endogenous MIF with anti-MIF antibody inhibited the activation of mouse spleen cells induced by TSST-1 in vitro and in vivo. These observations suggest that MIF is a necessary component of the mitogenic response of lymphocytes after activation with superantigenic staphylococcal exotoxins.
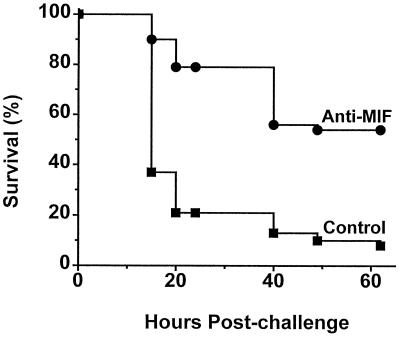
Anti-MIF Antibody Protects Mice from Lethal TSS.
Studies of experimental toxic shock induced by TSST-1 or by staphylococcal enterotoxin B in rabbits or mice have revealed a central role for TNFα and macrophage and T cell activation in the pathogenesis of these syndromes (16, 23, 24). The fact that TSST-1 and SPEA stimulate macrophages and splenocytes to produce MIF, that in turn is known to induce TNFα secretion by macrophages (8), suggested to us a potential role for MIF in the pathogenesis of TSSs. To test this hypothesis we developed a model of lethal TSST-1 shock in BALB/c mice sensitized with d-galactosamine. Treatment with anti-MIF antibody 2 hr before the injection of an otherwise lethal dose of TSST-1 increased survival from 8% to 54% (P = 0.00004, two-tailed χ2 test) (Fig. 5). Taken together with our previous observations that MIF is an important mediator of lethal endotoxemia, these findings implicate MIF as a critical cytokine of the host responses to both Gram-positive and Gram-negative infections and septic shock.
Figure 5.
Anti-MIF antibody protects mice from lethal toxic shock induced by TSST-1. BALB/c mice were injected i.p. with 200 μl of anti-MIF or nonimmune rabbit serum 2 hr before the i.p. injection of TSST-1 (1 mg/kg) and d-galactosamine (0.9 g/kg). Data points are from four independent experiments, each of which included 8 to 10 mice per treatment group. Survival was 54% (21 of 39) in mice treated with anti-MIF serum and 8% (3 of 38) in mice treated with nonimmune serum (P = 0.00004, two-tailed χ2 test).
DISCUSSION
In contrast to endotoxemia and Gram-negative septic shock, much less information is available concerning the pathophysiology of Gram-positive sepsis. In 1984, Ikejima et al. showed for the first time that filtrate from TSS-associated S. aureus strains induced IL-1 production by monocytes (13). TSST-1 was then found to stimulate TNFα and IL-1 secretion from human mononuclear cells and to cause a shock-like syndrome when injected in rabbits (14–16). Later, exotoxins of Gram-positive bacteria (the staphylococcal enterotoxins, exfoliating toxins or TSST-1, and the streptococcal pyrogenic exotoxins) were identified to be superantigens, acting to stimulate T cells subpopulations by cross-linking class II major histocompatibility complex proteins of antigen-presenting cells to particular Vβ chains of the T cell receptor (22, 25). Activation of macrophages and T cells leads to an outpouring of proinflammatory cytokines [TNFα, interleukin 1 (IL-1), lymphotoxin, IL-2, and IFNγ] (13–16, 20, 21), producing the fulminant clinical manifestations of the TSSs characterized by high fever, skin rash, cardiovascular collapse with severe and often prolonged shock, and multiorgan failure (26). Yet, many of the Gram-positive bacteria isolated from patients with septic shock do not produce toxins and thus cause shock by mechanisms that are largely unknown.
In the present report, we identify MIF to be critically involved in the activation of macrophages, pituitary cells and T cells by the Gram-positive exotoxins TSST-1 and SPEA. Both toxins induced MIF release from cultured macrophages and dose-response studies performed with mouse splenocytes showed that the threshold for MIF release after TSST-1 stimulation was inferior to that of the T cell cytokines IL-2 and IFNγ. These findings are in agreement with the results of previous studies that showed that concentrations of TSST-1 in the range of 0.1–1,000 ng/ml were required to induce IL-2 production by human peripheral blood or murine spleen lymphocytes, or IL-1 and TNF production by monocytes (13, 15, 20, 21). Taken together with the previously reported greater sensitivity of macrophage for releasing MIF compared with TNF in response to Gram-negative endotoxin (LPS) (8), the present study indicates that the threshold for MIF release by immune cells in response to stimulation by bacterial toxins is similar, if not inferior, to that of the pivotal cytokines TNF, IL-1, IL-2, or IFNγ. Given that MIF is released by both macrophages and T cells we did not seek to establish the secretory profile and amounts of MIF produced by each spleen cell type, particularly because the major histocompatibility complex class II proteins of antigen-presenting cells are required for activation of T lymphocytes by superantigens such as the staphylococcal enterotoxins or TSST-1 (25, 27–29).
The expression of MIF is tightly regulated. Whereas MIF can be induced by low concentrations of stimulant [e.g., TSST-1 and SPEA as shown in the present study, or endotoxin (LPS) (8) or glucocorticoids (6)], MIF production by macrophages and T cells follows a bell-shaped, concentration-dependent profile. High concentrations of TSST-1 or SPEA (>1 ng/ml), LPS (>100 ng/ml), or glucocorticoids (>10−7M) do not stimulate macrophage MIF release. Studies are in progress to identify the factors implicated either in a decreased synthesis or release of MIF or in an increased degradation of MIF at high toxin concentrations. The fact that MIF production is turned off at high concentrations of bacterial products may be an important protective mechanism of the host to prevent the harmful proinflammatory effects of MIF as shown in the TSST-1 shock model (Fig. 5) or during lethal endotoxemia (5).
Macrophage, pituitary, and splenocyte MIF secretion triggered by staphylococcal and streptococcal exotoxins is likely to be important in vivo. Activation of T cells, an important biological effect of bacterial superantigens, was observed to be markedly reduced after neutralization of MIF activity. In fact, pretreatment of mice with anti-MIF antibody before TSST-1 injection prevented spleen enlargement and reduced by 50% the proliferation of splenocytes measured ex vivo 72 hr after TSST-1 challenge (Fig. 4). The results therefore indicate that MIF contributes to the mitogenic response of lymphocytes after activation with staphylococcal exotoxins, confirming and extending our recent observation of a role for MIF in the activation of mouse T cells stimulated by anti-CD3 antibodies (10). The fact that macrophage and T cell cytokines play a pivotal role in the pathophysiology of TSS led us to explore the effect of MIF in an experimental toxic shock model that mimics human TSS (18). Neutralization of endogenous MIF with anti-MIF antibody produced a significant increase in survival in a lethal mouse model of TSST-1-induced shock (from 8% to 54%, P < 0.0001, Fig. 5). Of all the cytokines released by immune cells after exposure to Gram-positive exotoxins, TNFα has been shown to be a key mediator of TSS as neutralizing anti-TNF antibodies protected mice from shock induced by TSST-1 or staphylococcal enterotoxin B (23, 24). The present study thus also implicates MIF as an important mediator in the pathophysiology of TSS, a finding supported by our previous observation that MIF and TNF induces each other’s secretion from macrophages (8). It also re-emphasizes the critical role played by the macrophage in the hyperacute clinical manifestations and rapid downhill course of staphylococcal and streptococcal TSSs. Recent studies have ascribed a critical role for costimulatory molecules present on the membrane of antigen-presenting cells and their T cell counter-receptors in the pathogenesis of TSS (30, 31). CD28 knockout mice or mice treated with an CTLA4-Ig fusion protein were found to be resistant to TSST-1-induced shock. Interestingly, protection from death was associated with a nearly complete abrogation of the accumulation of serum TNFα and IFNγ, but not IL-2, indicating that CD28 and CTLA4 are necessary for the generation of these cytokines after TSST-1 stimulation. Whether MIF production by exotoxin-stimulated T cells also requires activation of the B7-CD28/CTLA4 pathway is unknown at the present time.
The expression of MIF induced both by proinflammatory stimuli and by glucocorticoids, and its capacity to control glucocorticoid-mediated suppression of cytokine production, has placed this mediator in a central position within the inflammatory and immune cell responses (6, 8, 32). MIF is released early after cell stimulation and much of this release response can be attributed to the rapid secretion of MIF protein from preformed granular or cytoplasmic pools (7). Within that context it is also significant that MIF is released into the circulation centrally, by the pituitary gland, and peripherally, at the site of inflammation (32). MIF production by the pituitary gland and immune cells during the course of the host response to infection is vital for the development of an ensuing inflammatory and immune response. This has been borne out in vivo by the observation that recombinant MIF potentiates the endotoxic shock response (5) and overrides the protective effects of glucocorticoids (6, 10, 11), and by studies in which MIF neutralization protects mice from lethal endotoxemia (5), decreases the delayed-type hypersensitivity response (33), and inhibits the generation of antigen-specific T cells and humoral antibody (10). More recent experiments have shown that anti-MIF antibodies also decrease significantly the inflammation associated with a rat model of glomerulonephritis (34), murine models of arthritis (35), allograft rejection (C.N.M. and R.B., unpublished observations), and E. coli peritonitis (T.C., unpublished observations).
Taken together with our previous observations that MIF is an important mediator of lethal endotoxemia (5), the results of the present study assign a critical role for MIF in the innate immune response to microbial products. Conceivably, anti-MIF strategies may someday find utility in the management of the TSSs and other clinical conditions in which the cytokine balance is shifted toward excessive inflammation and tissue damage.
Acknowledgments
T.C. is supported by a career award from the Swiss National Science Foundation (32-48916.96 and 32-49129.96). This work was supported by a grant from the Fonds de perfectionnement du Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland and the National Institutes of Health AI35931 (R.B.).
ABBREVIATIONS
- MIF
macrophage migration inhibitory factor
- LPS
lipopolysaccharide
- SPEA
streptococcal pyrogenic exotoxin A
- TSS
toxic-shock syndrome
- TSST-1
TSS toxin 1
- IL
interleukin
- IFN
interferon
- TNF
tumor necrosis factor
References
- 1.Bloom B R, Bennett B. Science. 1966;153:80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 2.David J. Proc Natl Acad Sci USA. 1966;56:72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan C F, Remold H G, David J R. J Exp Med. 1973;137:275–288. doi: 10.1084/jem.137.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan C F, Karnovsky M L, David J R. J Exp Med. 1971;133:1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernhagen J, Calandra T, Mitchell R A, Martin S B, Tracey K J, Voelter W, Manogue K R, Cerami A, Bucala R. Nature (London) 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 6.Calandra T, Bernhagen J, Metz C N, Spiegel L A, Bacher M, Donnelly T, Cerami A, Bucala R. Nature (London) 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 7.Nishino T, Bernhagen J, Shiiki H, Calandra T, Dohi K, Bucala R. Mol Med. 1995;1:781–788. [PMC free article] [PubMed] [Google Scholar]
- 8.Calandra T, Bernhagen J, Mitchell R A, Bucala R. J Exp Med. 1994;179:1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernhagen J, Mitchell R A, Calandra T, Voelter W, Cerami A, Bucala R. Biochemistry. 1994;33:14144–14155. doi: 10.1021/bi00251a025. [DOI] [PubMed] [Google Scholar]
- 10.Bacher M, Metz C N, Calandra T, Mayer K, Chesney J, Lohoff M, Gemsa D, Donnelly T, Bucala R. Proc Natl Acad Sci USA. 1996;93:7849–7854. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donelly S C, Haslett C, Reid P T, Grant I S, Wallace W A H, Metz C N, Bruce L J, Bucala R. Nat Med. 1997;3:320–323. doi: 10.1038/nm0397-320. [DOI] [PubMed] [Google Scholar]
- 12.Bone R C. Arch Intern Med. 1994;154:26–34. [PubMed] [Google Scholar]
- 13.Ikejima T, Dinarello C A, Gill M, Wolff S M. J Clin Invest. 1984;73:1312–1320. doi: 10.1172/JCI111334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsonnet J, Hickman R K, Eardley D D, Pier G B. J Infect Dis. 1985;151:514–522. doi: 10.1093/infdis/151.3.514. [DOI] [PubMed] [Google Scholar]
- 15.Jupin C, Anderson S, Damais C, Alouf J E, Parant M. J Exp Med. 1988;167:752–761. doi: 10.1084/jem.167.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikejima T, Okusawa S, Van der Meer J W M, Dinarello C A. J Infect Dis. 1988;158:1017–1025. doi: 10.1093/infdis/158.5.1017. [DOI] [PubMed] [Google Scholar]
- 17.Scherer M T, Ignatowicz L, Winslow G M, Kappler J W, Marrack P. Annu Rev Cell Biol. 1993;9:101–128. doi: 10.1146/annurev.cb.09.110193.000533. [DOI] [PubMed] [Google Scholar]
- 18.Miethke T, Wahl C, Regele D, Gaus H, Heeg K, Wagner H. Immunobiology. 1993;189:270–284. doi: 10.1016/S0171-2985(11)80362-1. [DOI] [PubMed] [Google Scholar]
- 19.Kruisbeek A M. In: In Vitro Assays for Mouse Lymphocyte Function. Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. 1993. pp. (3)3.1.3–3.1.5. [Google Scholar]
- 20.Micusan V V, Mercier G, Bhatti A R, Reiser R F, Bergdoll M S, Oth D. Immunology. 1986;58:203–208. [PMC free article] [PubMed] [Google Scholar]
- 21.Uchiyama T, Kamagata Y, Wakai M, Yoshioka M, Fujikama H, Igarashi H. Microbiol Immunol. 1986;30:469–483. doi: 10.1111/j.1348-0421.1986.tb02973.x. [DOI] [PubMed] [Google Scholar]
- 22.Marrack P, Kappler J. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 23.Miethke T, Wahl C, Heeg K, Echtenacher B, Krammer P H, Wagner H. J Exp Med. 1992;175:91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miethke T, Duschek K, Wahl C, Heeg K, Wagner H. Eur J Immunol. 1993;23:1494–1500. doi: 10.1002/eji.1830230715. [DOI] [PubMed] [Google Scholar]
- 25.Kappler J, Kotzin B, Herron L, Gelfand E W, Bigler R D, Boylston A, Carrel S, Posnett D N, Choi Y, Marrack P. Science. 1989;244:811–813. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- 26.Todd, J., Fishaut, M., Kapral, F. & Welch, T. (1978) Lancet, 1116–1118. [DOI] [PubMed]
- 27.Fleischer B, Schrezenmeir H. J Exp Med. 1988;167:1697–1707. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mollick J A, Cook R G, Rich R R. Science. 1989;244:817–820. doi: 10.1126/science.2658055. [DOI] [PubMed] [Google Scholar]
- 29.Fraser J D. Nature (London) 1989;339:221–223. doi: 10.1038/339221a0. [DOI] [PubMed] [Google Scholar]
- 30.Saha B, Harlan D M, Lee K P, June C H, Abe R. J Exp Med. 1996;183:2675–2680. doi: 10.1084/jem.183.6.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saha B, Jaklic B, Harlan D M, Gray G S, June C H, Abe R. J Immunol. 1996;157:3869–3875. [PubMed] [Google Scholar]
- 32.Calandra T, Bucala R. Crit Rev Immunol. 1997;17:77–88. doi: 10.1615/critrevimmunol.v17.i1.30. [DOI] [PubMed] [Google Scholar]
- 33.Bernhagen J, Bacher M, Calandra T, Metz C N, Doty S B, Donnelly T, Bucala R. J Exp Med. 1996;183:277–282. doi: 10.1084/jem.183.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lan H Y, Bacher M, Yang N, Mu W, Nikolic-Paterson D J, Metz C, Meinhardt A, Bucala R, Atkins R C. J Exp Med. 1997;185:1455–1465. doi: 10.1084/jem.185.8.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikulowska A, Metz C N, Bucala R, Holmdahl R. J Immunol. 1997;158:5514–5517. [PubMed] [Google Scholar]