Abstract
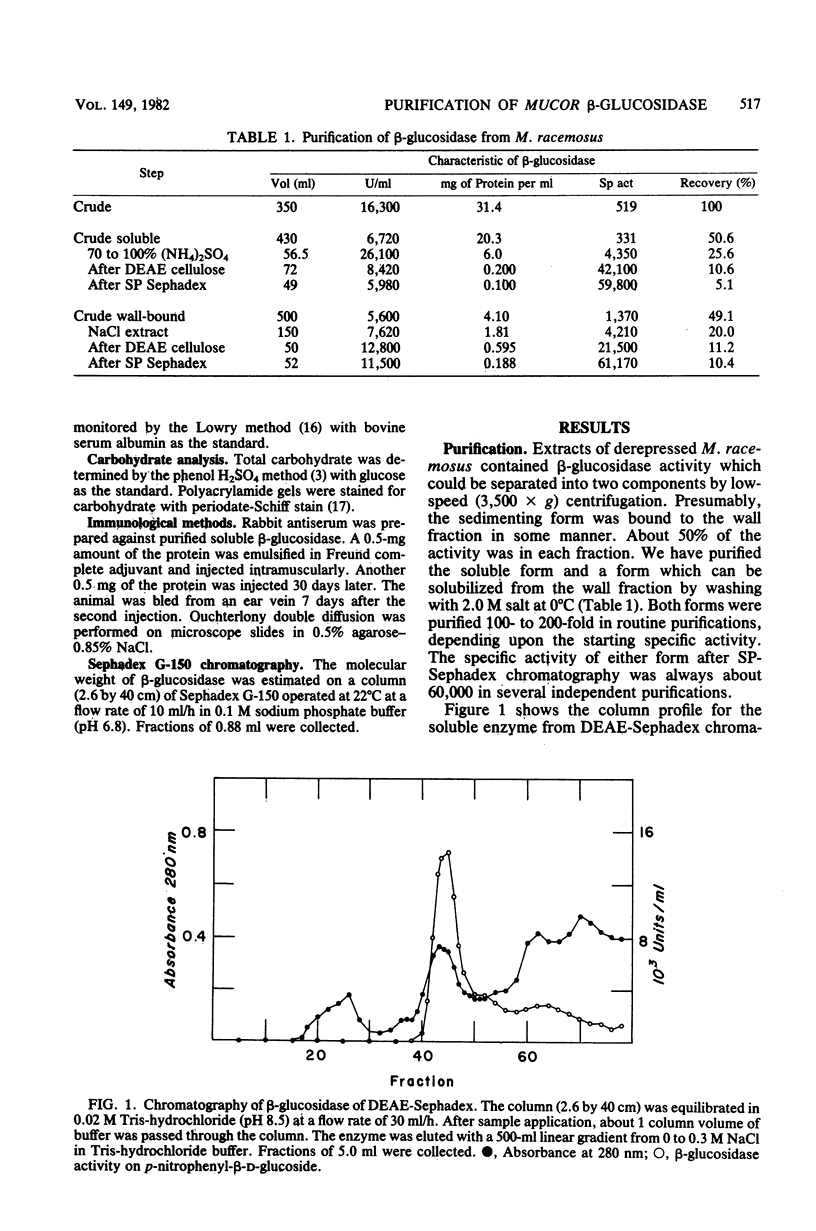
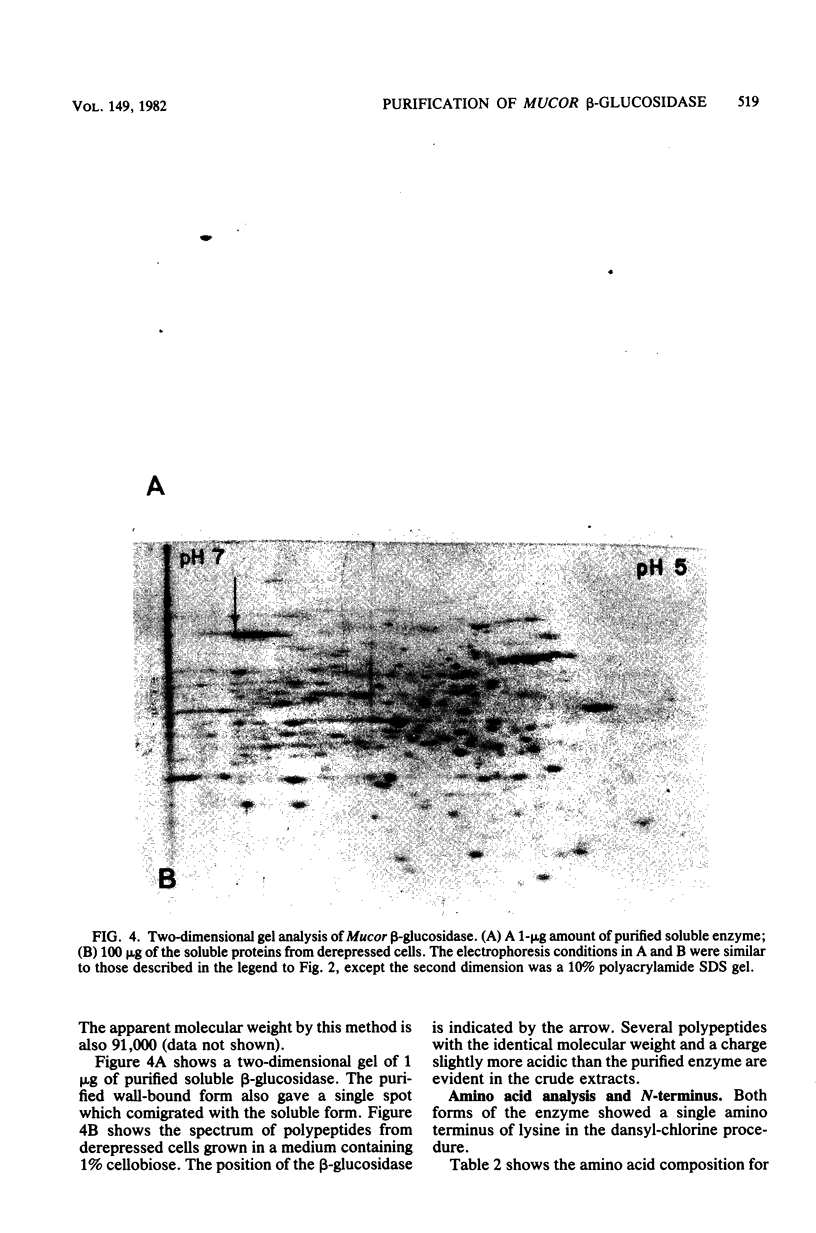
beta-Glucosidase activity in crude extracts of Mucor racemosus exists in a soluble form and in a wall-bound form which sediments at 3,500 x g. The soluble form and a wall-bound form were purified to homogeneity by ammonium sulfate fractionation. DEAE-Sephadex chromatography, and SP-Sephadex chromatography. Both forms were identical in all parameters measured. Each enzyme is a glycoprotein of 91,000 daltons, with an identical amino acid composition and N-terminal amino acid of lysine; both contain about 10% carbohydrate. Both forms catalyze the hydrolysis of cellobiose and p-nitrophenyl-beta-D-glucoside with identical kinetic constants.
Full text
PDF




Images in this article
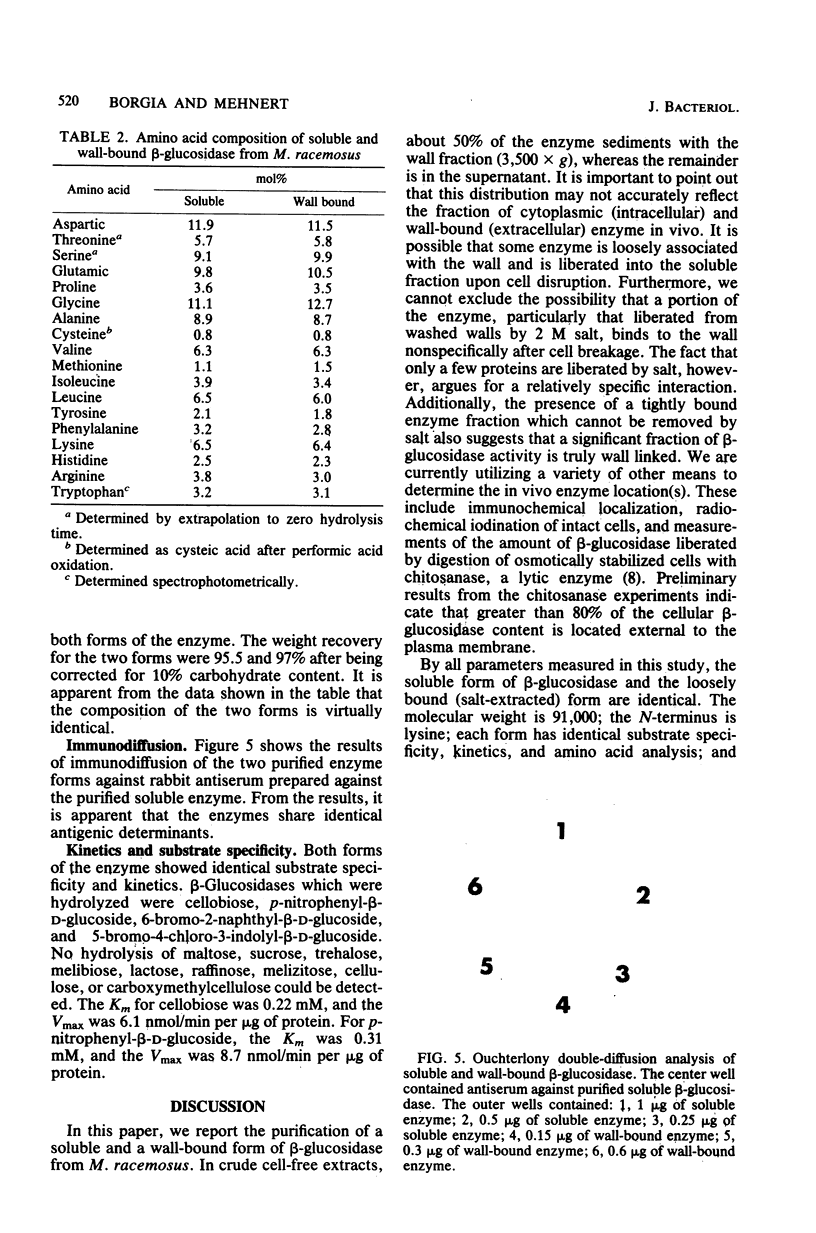
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTNICKI-GARCIA S., NICKERSON W. J. Nutrition, growth, and morphogenesis of Mucor rouxii. J Bacteriol. 1962 Oct;84:841–858. doi: 10.1128/jb.84.4.841-858.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgia P., Sypherd P. S. Control of beta-glucosidase synthesis in Mucor racemosus. J Bacteriol. 1977 May;130(2):812–817. doi: 10.1128/jb.130.2.812-817.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart B. M., Beck R. S. Localization of the beta-glucosidases in Neurospora crassa. J Bacteriol. 1970 Feb;101(2):408–417. doi: 10.1128/jb.101.2.408-417.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Flores-Carreon A., Reyes E., Ruiz-Herrera J. Inducible cell wall-bound alpha-glucosidase in Mucor rouxii. Biochim Biophys Acta. 1970 Nov 24;222(2):354–360. doi: 10.1016/0304-4165(70)90124-8. [DOI] [PubMed] [Google Scholar]
- Flores-Carreón A., Reyes E., Ruíz-Herrera J. Influence of oxygen on maltose metabolism by Mucor rouxii. J Gen Microbiol. 1969 Nov;59(1):13–19. doi: 10.1099/00221287-59-1-13. [DOI] [PubMed] [Google Scholar]
- Genthner F. J., Borgia P. T. Spheroplast fusion and heterokaryon formation in Mucor racemosus. J Bacteriol. 1978 Apr;134(1):349–352. doi: 10.1128/jb.134.1.349-352.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzner H. G. Cell wall alterations associated with the hyperproduction of extracellular enzymes in Neurospora crassa. J Bacteriol. 1972 Aug;111(2):443–446. doi: 10.1128/jb.111.2.443-446.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros C., Labouesse B. Study of the dansylation reaction of amino acids, peptides and proteins. Eur J Biochem. 1969 Feb;7(4):463–470. doi: 10.1111/j.1432-1033.1969.tb19632.x. [DOI] [PubMed] [Google Scholar]
- HILL E. P., SUSSMAN A. S. DEVELOPMENT OF TREHALASE AND INVERTASE ACTIVITY IN NEUROSPORA. J Bacteriol. 1964 Dec;88:1556–1566. doi: 10.1128/jb.88.6.1556-1566.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks D. L., Sussman A. S. The relation between growth, conidiation and trehalase activity in Neurospora crassa. Am J Bot. 1969 Nov-Dec;56(10):1152–1159. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampen J. O. External enzymes of yeast: their nature and formation. Antonie Van Leeuwenhoek. 1968;34(1):1–18. doi: 10.1007/BF02046409. [DOI] [PubMed] [Google Scholar]
- Larsen A. D., Sypherd P. S. Cyclic adenosine 3',5'-monophosphate and morphogenesis in Mucor racemosus. J Bacteriol. 1974 Feb;117(2):432–438. doi: 10.1128/jb.117.2.432-438.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METZENBERG R. L. THE LOCALIZATION OF BETA-FRUCTOFURANOSIDASE IN NEUROSPORA. Biochim Biophys Acta. 1963 Nov 8;77:455–465. doi: 10.1016/0006-3002(63)90521-3. [DOI] [PubMed] [Google Scholar]
- Neumann N. P., Lampen J. O. Purification and properties of yeast invertase. Biochemistry. 1967 Feb;6(2):468–475. doi: 10.1021/bi00854a015. [DOI] [PubMed] [Google Scholar]
- Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pedersen S., Reeh S. V. Analysis of the proteins synthesized in ultraviolet light-irradiated Escherichia coli following infection with the bacteriophages lambdadrifd 18 and lambdadfus-3. Mol Gen Genet. 1976 Mar 30;144(3):339–343. doi: 10.1007/BF00341733. [DOI] [PubMed] [Google Scholar]
- Peters J., Sypherd P. S. Morphology-associated expression nicotinamide adenine dinucleotide-dependent glutamate dehydrogenase in Mucorracemosus. J Bacteriol. 1979 Mar;137(3):1134–1139. doi: 10.1128/jb.137.3.1134-1139.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes E., Ruiz-Herrera J. Mechanism of maltose utilization by Mucor rouxii. Biochim Biophys Acta. 1972 Jul 19;273(2):328–335. doi: 10.1016/0304-4165(72)90224-3. [DOI] [PubMed] [Google Scholar]
- Smith W. L., Ballou C. E. Immunochemical characterization of the mannan component of the external invertase (beta-fructofuranosidase) of Saccharomyces cerevisiae. Biochemistry. 1974 Jan 15;13(2):355–361. doi: 10.1021/bi00699a021. [DOI] [PubMed] [Google Scholar]
- Sorrentino A. P., Zorzópulos J., Terenzi H. F. Inducible alpha-glucosidase of Mucor rouxii. Effects of dimorphism on the development of the wall-bound activity. Arch Biochem Biophys. 1977 Apr 30;180(2):232–238. doi: 10.1016/0003-9861(77)90033-9. [DOI] [PubMed] [Google Scholar]