Abstract
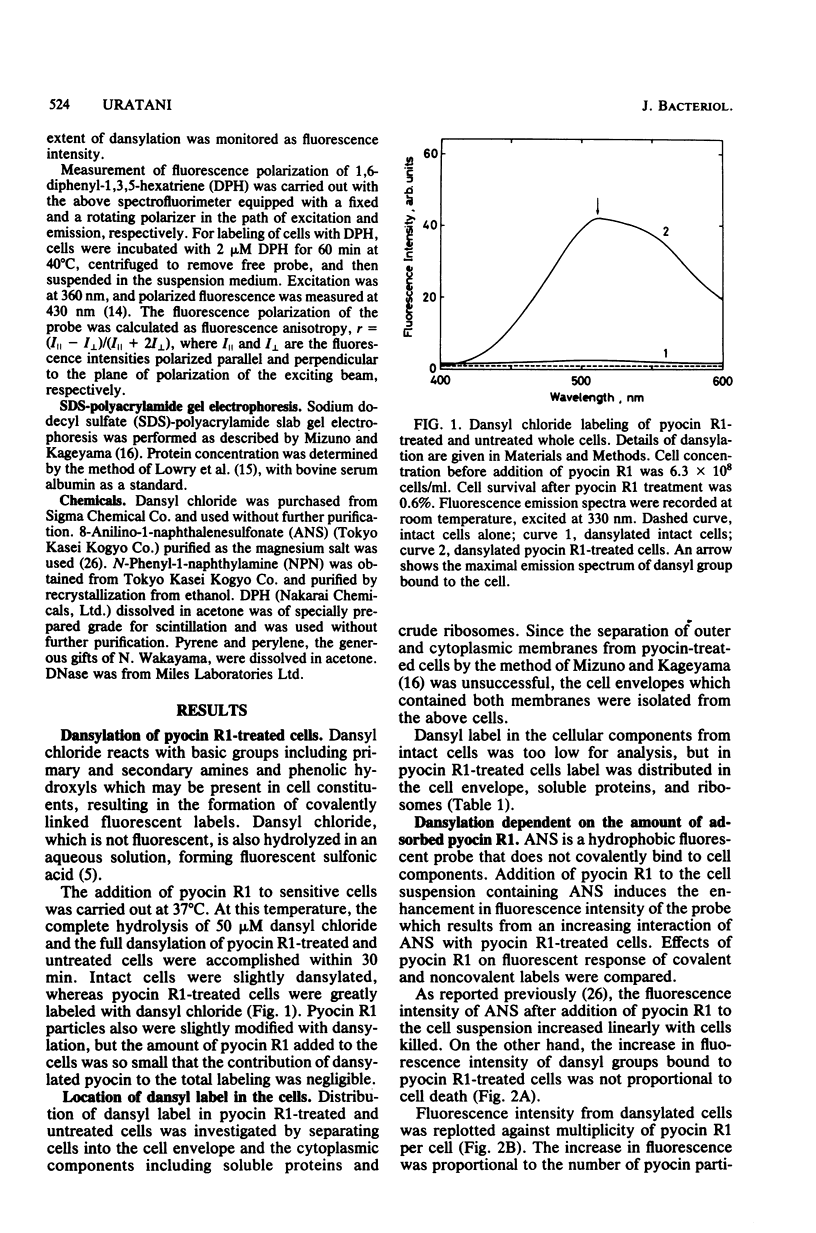
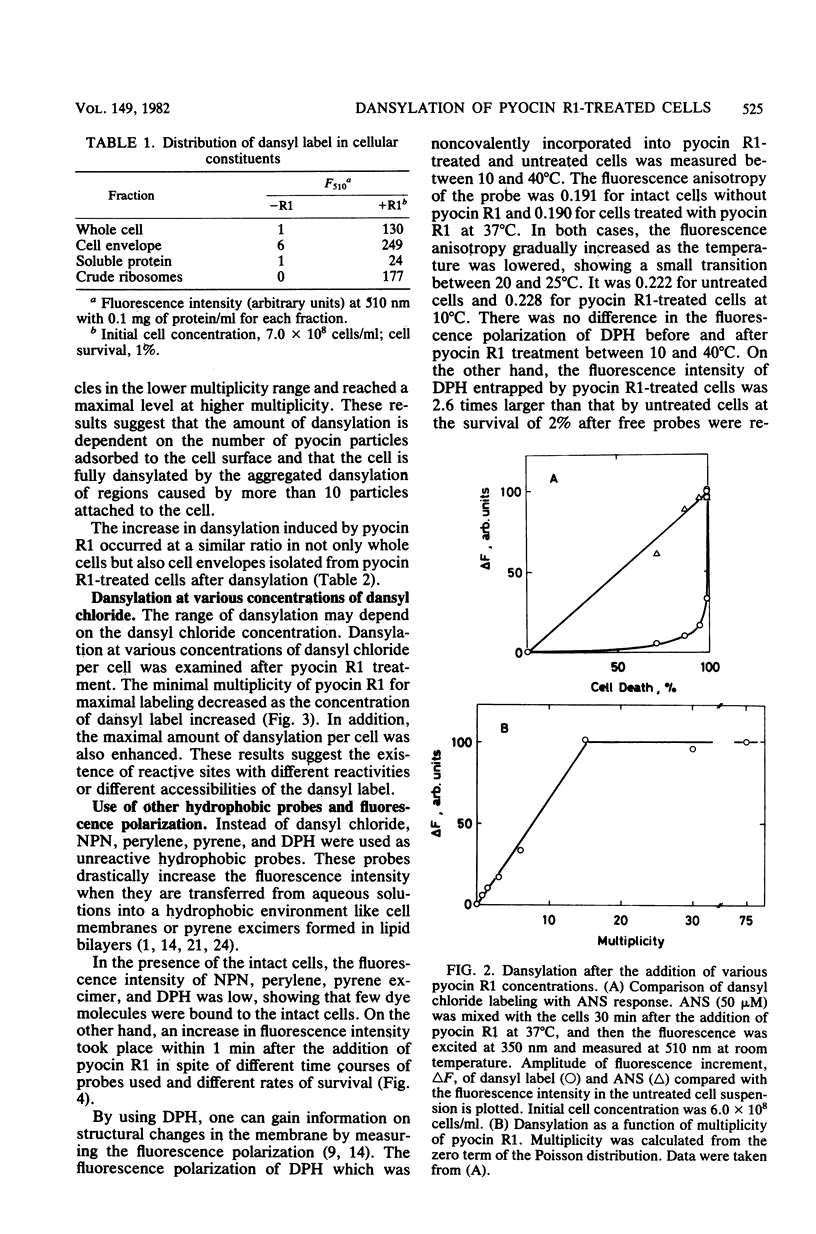
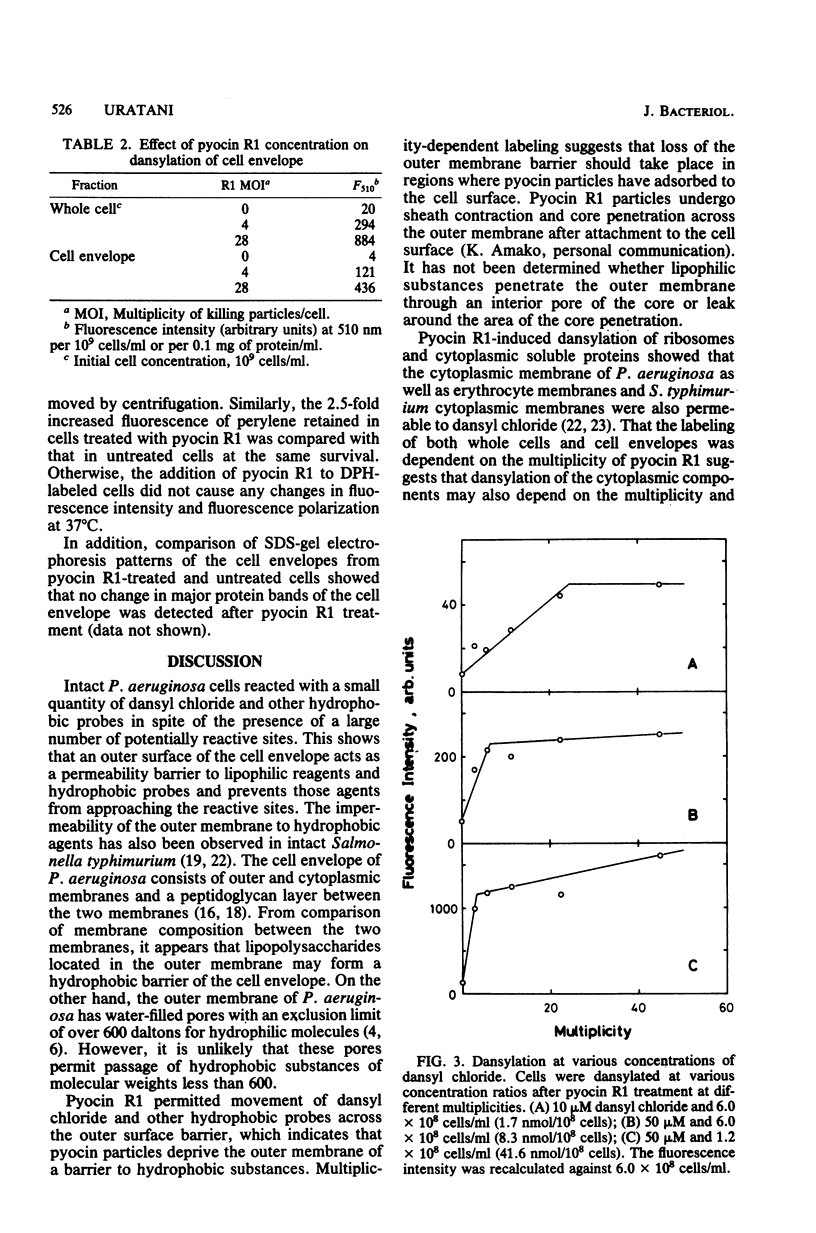
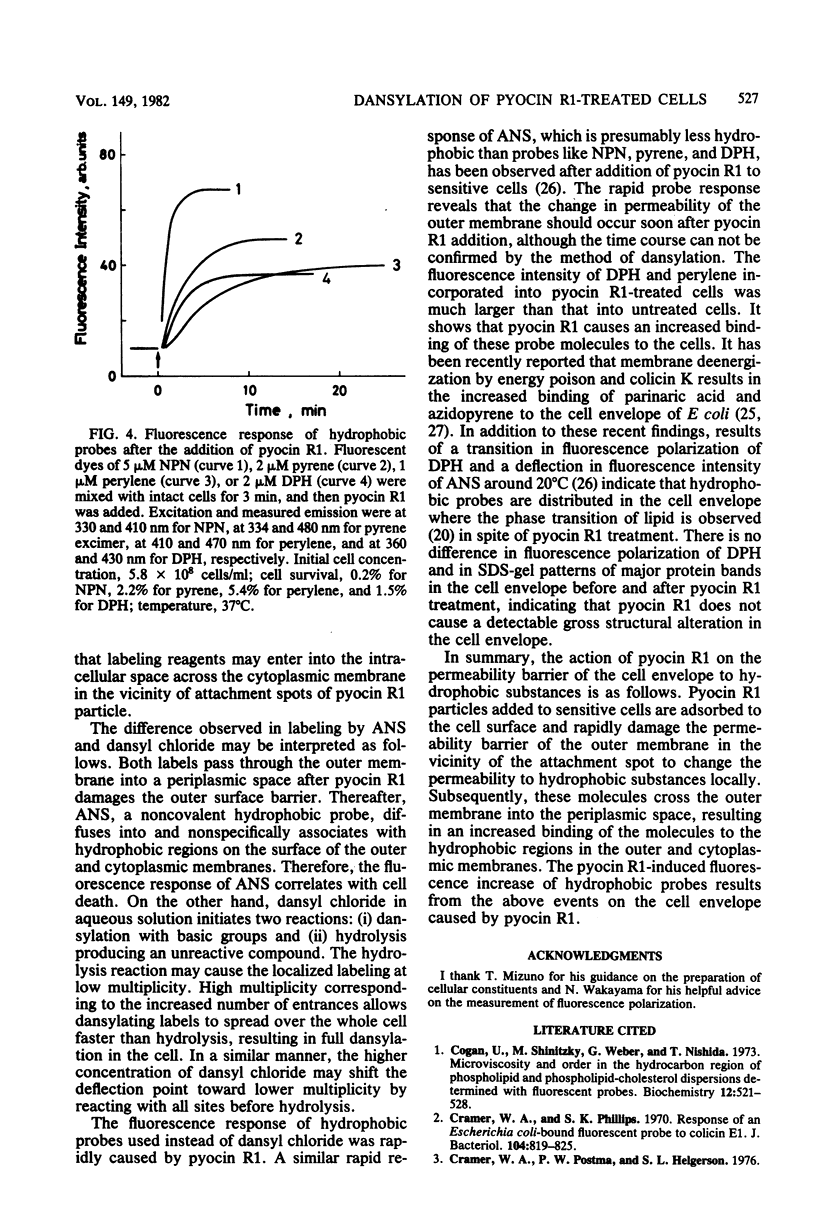
Pyocin R1, a bacteriocin of Pseudomonas aeruginosa, caused an increase in binding of fluorescent label, 1-dimethylaminonaphthalene-5-sulfonyl chloride (dansyl chloride), to sensitive cells. In pyocin R1-treated cells, cytoplasmic soluble proteins and crude ribosomes as well as cell envelopes were labeled by dansyl chloride. The amount of bound dye was proportional to the multiplicity of pyocin R1 and reached a maximal level at high multiplicity. In addition, pyocin R1 rapidly caused an increase in fluorescence intensity of the hydrophobic probes N-phenyl-1-naphthylamine, pyrene, and perylene, which were mixed with cells. These results show that pyocin R1 damages locally a cell envelope barrier to hydrophobic solutes and allows dyes to penetrate into the intracellular space across the barrier.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cogan U., Shinitzky M., Weber G., Nishida T. Microviscosity and order in the hydrocarbon region of phospholipid and phospholipid-cholesterol dispersions determined with fluorescent probes. Biochemistry. 1973 Jan 30;12(3):521–528. doi: 10.1021/bi00727a026. [DOI] [PubMed] [Google Scholar]
- Cramer W. A., Phillips S. K. Response of an Escherichia coli-bound fluorescent probe to colicin E1. J Bacteriol. 1970 Nov;104(2):819–825. doi: 10.1128/jb.104.2.819-825.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer W. A., Postma P. W., Helgerson S. L. An evaluation of N-phenyl-1-naphthylamine as a probe of membrane energy state in Escherichia coli. Biochim Biophys Acta. 1976 Dec 6;449(3):401–411. doi: 10.1016/0005-2728(76)90151-1. [DOI] [PubMed] [Google Scholar]
- Decad G. M., Nikaido H. Outer membrane of gram-negative bacteria. XII. Molecular-sieving function of cell wall. J Bacteriol. 1976 Oct;128(1):325–336. doi: 10.1128/jb.128.1.325-336.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M. Mode of action of pyocin R1. J Biochem. 1978 Feb;83(2):395–402. doi: 10.1093/oxfordjournals.jbchem.a131926. [DOI] [PubMed] [Google Scholar]
- Ishii S. I., Nishi Y., Egami F. The fine structure of a pyocin. J Mol Biol. 1965 Sep;13(2):428–431. doi: 10.1016/s0022-2836(65)80107-3. [DOI] [PubMed] [Google Scholar]
- KAGEYAMA M., IKEDA K., EGAMI F. STUDIES OF A PYOCIN. III. BIOLOGICAL PROPERTIES OF THE PYOCIN. J Biochem. 1964 Jan;55:59–64. doi: 10.1093/oxfordjournals.jbchem.a127841. [DOI] [PubMed] [Google Scholar]
- KAGEYAMA M. STUDIES OF A PYOCIN. I. PHYSICAL AND CHEMICAL PROPERTIES. J Biochem. 1964 Jan;55:49–53. doi: 10.1093/oxfordjournals.jbchem.a127839. [DOI] [PubMed] [Google Scholar]
- Kageyama M. Effect of pyocin R1 on the glucose metabolism of sensitive cells of Pseudomonas aeruginosa. J Biochem. 1978 Dec;84(6):1373–1379. doi: 10.1093/oxfordjournals.jbchem.a132259. [DOI] [PubMed] [Google Scholar]
- Kaziro Y., Tanaka M. Studies on the mode of action of pyocin. I. Inhibition of macromolecular synthesis in sensitive cells. J Biochem. 1965 May;57(5):689–695. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lentz B. R., Barenholz Y., Thompson T. E. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 1. Single component phosphatidylcholine liposomes. Biochemistry. 1976 Oct 5;15(20):4521–4528. doi: 10.1021/bi00665a029. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J Biochem. 1978 Jul;84(1):179–191. doi: 10.1093/oxfordjournals.jbchem.a132106. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Mizushima S. Characterization of stuck ribosomes induced by neomycin in vivo and in vitro. Biochim Biophys Acta. 1974 Jun 14;353(1):69–76. doi: 10.1016/0005-2787(74)90098-7. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Overath P., Brenner M., Gulik-Krzywicki T., Shechter E., Letellier L. Lipid phase transitions in cytoplasmic and outer membranes of Escherichia coli. Biochim Biophys Acta. 1975 May 6;389(2):358–369. doi: 10.1016/0005-2736(75)90328-4. [DOI] [PubMed] [Google Scholar]
- Phillips S. K., Cramer W. A. Properties of the fluorescence probe response associated with the transmission mechanism of colicin E1. Biochemistry. 1973 Mar 13;12(6):1170–1176. doi: 10.1021/bi00730a024. [DOI] [PubMed] [Google Scholar]
- Schindler P. R., Teuber M. Ultrastructural study of Salmonella typhimurium treated with membrane-active agents: specific reaction dansylchloride with cell envelope components. J Bacteriol. 1978 Jul;135(1):198–206. doi: 10.1128/jb.135.1.198-206.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Knüfermann H., Wallach D. F. The reaction of 1-dimethylaminonaphthalene-5-sulfonyl chloride (DANSC1) with erythrocyte membranes. A new look at "vectorial" membrane probes. Biochim Biophys Acta. 1973 May 11;307(2):353–365. doi: 10.1016/0005-2736(73)90101-6. [DOI] [PubMed] [Google Scholar]
- Soutar A. K., Pownall H. J., Hu A. S., Smith L. C. Phase transitions in bilamellar vesicles. Measurements by pyrene excimer fluorescence and effect on transacylation by lecithin: cholesterol acyltransferase. Biochemistry. 1974 Jul 2;13(14):2828–2836. doi: 10.1021/bi00711a008. [DOI] [PubMed] [Google Scholar]
- Tecoma E. S., Wu D. Membrane deenergization by colicin K affects fluorescence of exogenously added but not biosynthetically esterified parinaric acid probes in Escherichia coli. J Bacteriol. 1980 Jun;142(3):931–938. doi: 10.1128/jb.142.3.931-938.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uratani Y., Kageyama M. A fluorescent probe response to the interaction of pyocin R1 with sensitive cells. J Biochem. 1977 Feb;81(2):333–341. doi: 10.1093/oxfordjournals.jbchem.a131463. [DOI] [PubMed] [Google Scholar]
- Wolf M. K., Konisky J. Increased binding of a hydrophobic, photolabile probe to Escherichia coli inversely correlates to membrane potential but not adenosine 5'-triphosphate levels. J Bacteriol. 1981 Jan;145(1):341–347. doi: 10.1128/jb.145.1.341-347.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



