Abstract
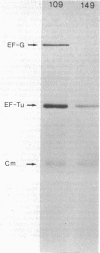
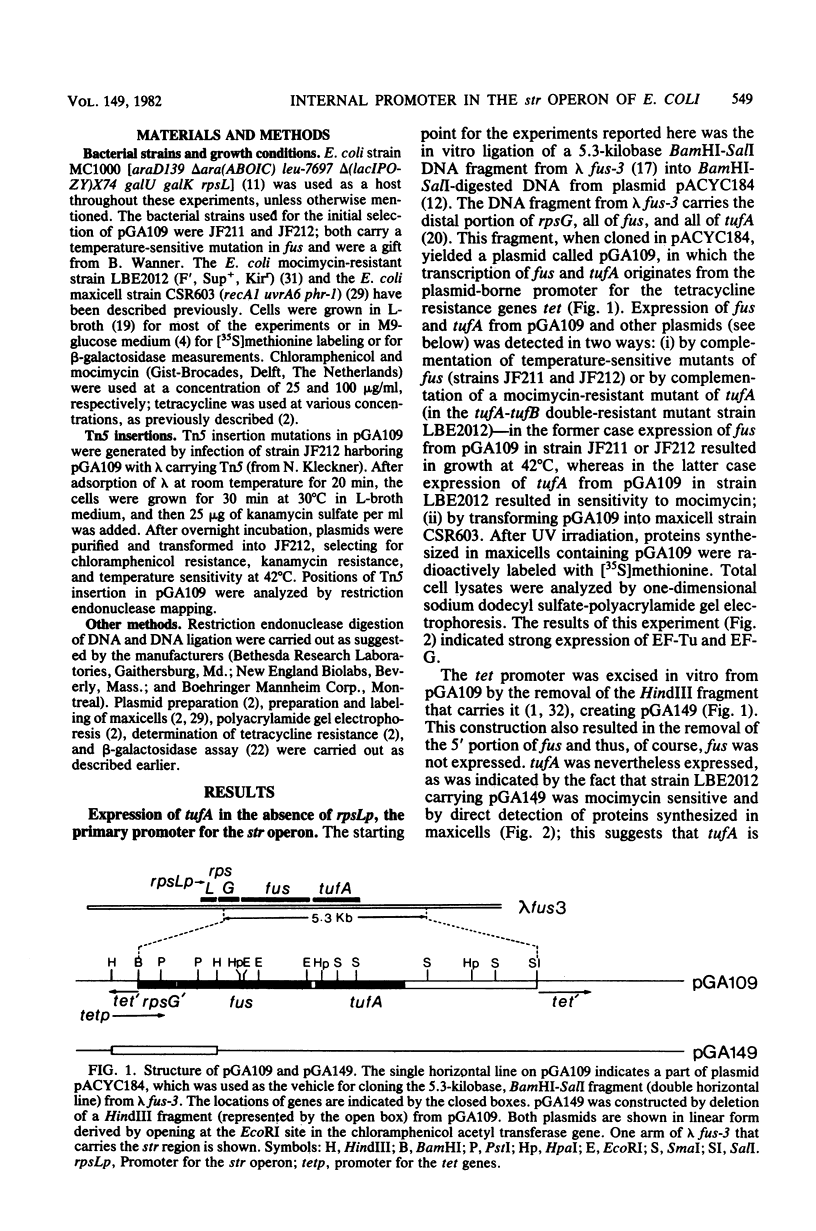
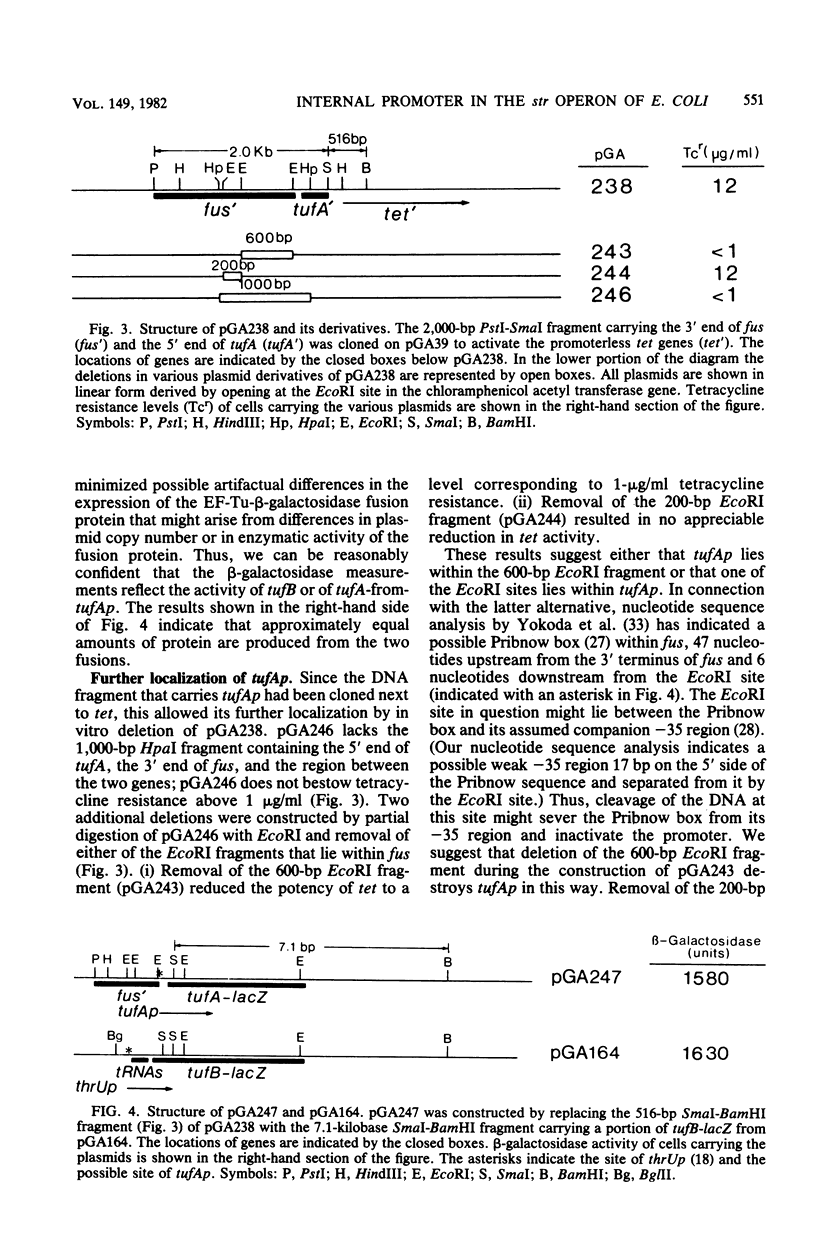
We constructed plasmids carrying tufA from which the major promoter for the rpsL-rpsG-fus-tufA operon (also called the str operon) had been removed. These plasmids continued to express tufA, as judged by the ability to complement mocimycin resistance and by electrophoretic analysis of synthesized proteins. Tn5 transpositions into fus, the gene for elongation factor G, which lies immediately on the 5' side of tufA, failed to obstruct the expression of tufA. The subcloning of a 2,000-base-pair PstI-SmaI DNA fragment (containing the intercistronic region between tufA and fus, the distal portion of fus, and the proximal portion of tufA) next to promoterless tetracycline resistance genes (tet) yielded a plasmid that was capable of bestowing resistance to 12 microgram of tetracycline per ml. The removal of an EcoRI fragment that lies within fus destroyed the ability of the 2,000-base-pair PstI-SmaI fragment to promote the transcription of tet. These data indicate that, in addition to the operon's major promoter rpsLp, there is an internal promoter, tufAp, which can be used for the transcription of tufA, tufAp probably lies within fus, about 50 base pairs upstream from its 3' end and 120 base pairs from the start codon of tufA. The relative activities of tufB and of tufA-from-tufAp were estimated by a comparison of beta-galactosidase activities of almost identical EF-Tu-beta-galactosidase protein fusions; they were approximately equal.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Friesen J. D. Characterization of promoter-cloning plasmids: analysis of operon structure in the rif region of Escherichia coli and isolation of an enhanced internal promoter mutant. J Bacteriol. 1980 Dec;144(3):904–916. doi: 10.1128/jb.144.3.904-916.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An G., Friesen J. D. Plasmid vehicles for direct cloning of Escherichia coli promoters. J Bacteriol. 1979 Nov;140(2):400–407. doi: 10.1128/jb.140.2.400-407.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An G., Friesen J. D. The nucleotide sequence of tufB and four nearby tRNA structural genes of Escherichia coli. Gene. 1980 Dec;12(1-2):33–39. doi: 10.1016/0378-1119(80)90013-x. [DOI] [PubMed] [Google Scholar]
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K., Clark B. F., Duffy L., Jones M. D., Kaziro Y., Laursen R. A., L'Italien J., Miller D. L., Nagarkatti S., Nakamura S. Primary structure of elongation factor Tu from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1326–1330. doi: 10.1073/pnas.77.3.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G., Squires C. L., Squires C. Control features within the rplJL-rpoBC transcription unit of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4922–4926. doi: 10.1073/pnas.76.10.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G., Squires C., Squires C. L. Attenuation and processing of RNA from the rplJL--rpoBC transcription unit of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3331–3335. doi: 10.1073/pnas.77.6.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Weiss A., Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980 May;142(2):439–446. doi: 10.1128/jb.142.2.439-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P. Transcription patterns of adjacent segments on the chromosome of Escherichia coli containing genes coding for four 50S ribosomal proteins and the beta and beta' subunits of RNA polymerase. J Mol Biol. 1977 Oct 5;115(4):603–625. doi: 10.1016/0022-2836(77)90105-x. [DOI] [PubMed] [Google Scholar]
- Furano A. V. Content of elongation factor Tu in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4780–4784. doi: 10.1073/pnas.72.12.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furano A. V. The elongation factor Tu coded by the tufA gene of Escherichia coli K-12 is almost identical to that coded by the tufB gene. J Biol Chem. 1977 Mar 25;252(6):2154–2157. [PubMed] [Google Scholar]
- Gegenheimer P., Apirion D. Structural characterization and in vitro processing of Escherichia coli ribosomal RNA transcripts containing 5- triphosphates, leader sequences, 16 S rRNA, and spacer tRNAs. J Mol Biol. 1980 Nov 5;143(3):227–257. doi: 10.1016/0022-2836(80)90188-6. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Identification of two copies of the gene for the elongation factor EF-Tu in E. coli. Nature. 1975 Oct 9;257(5526):458–462. doi: 10.1038/257458a0. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lee J. S., An G., Friesen J. D., Fill N. P. Location of the tufB promoter of E. coli: cotranscription of tufB with four transfer RNA genes. Cell. 1981 Jul;25(1):251–258. doi: 10.1016/0092-8674(81)90250-6. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Post L., Zengel J., Gilbert S. F., Strycharz W. A., Nomura M. Mapping of ribosomal protein genes by in vitro protein synthesis using DNA fragments of lambdafus3 transducing phage DNA as templates. J Biol Chem. 1977 Oct 25;252(20):7365–7383. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nomura M., Morgan E. A. Genetics of bacterial ribosomes. Annu Rev Genet. 1977;11:297–347. doi: 10.1146/annurev.ge.11.120177.001501. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Blumenthal R. M., Reeh S., Russell L. B., Lemaux P., Laursen R. A., Nagarkatti S., Friesen J. D. A mutant of Escherichia coli with an altered elongation factor Tu. Proc Natl Acad Sci U S A. 1976 May;73(5):1698–1701. doi: 10.1073/pnas.73.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. E., Arfsten A. E., Nomura M., Jaskunas S. R. Isolation and characterization of a promoter mutant in the str ribosomal protein operon in E. coli. Cell. 1978 Sep;15(1):231–236. doi: 10.1016/0092-8674(78)90097-1. [DOI] [PubMed] [Google Scholar]
- Pribnow D. Bacteriophage T7 early promoters: nucleotide sequences of two RNA polymerase binding sites. J Mol Biol. 1975 Dec 15;99(3):419–443. doi: 10.1016/s0022-2836(75)80136-7. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. E., Burgess R. R. Escherichia coli RNA polymerase binding and initiation of transcription on fragments of lambda rifd 18 DNA containing promoters for lambda genes and for rrnB, tufB, rplC,A, rplJ,L, and rpoB,C genes. Gene. 1979 Aug;6(4):331–365. doi: 10.1016/0378-1119(79)90073-8. [DOI] [PubMed] [Google Scholar]
- Van de Klundert J. A., Van der Meide P. H., Van de Putte P., Bosch L. Mutants of Escherichia coli altered in both genes coding for the elongation factor Tu. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4470–4473. doi: 10.1073/pnas.75.9.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widera G., Gautier F., Lindenmaier W., Collins J. The expression of tetracycline resistance after insertion of foreign DNA fragments between the EcoRI and HindIII sites of the plasmid cloning vector pBR 322. Mol Gen Genet. 1978 Jul 25;163(3):301–305. doi: 10.1007/BF00271959. [DOI] [PubMed] [Google Scholar]
- Yokota T., Sugisaki H., Takanami M., Kaziro Y. The nucleotide sequence of the cloned tufA gene of Escherichia coli. Gene. 1980 Dec;12(1-2):25–31. doi: 10.1016/0378-1119(80)90012-8. [DOI] [PubMed] [Google Scholar]
- Young F. S., Furano A. V. Regulation of the synthesis of E. coli elongation factor Tu. Cell. 1981 Jun;24(3):695–706. doi: 10.1016/0092-8674(81)90096-9. [DOI] [PubMed] [Google Scholar]