Abstract
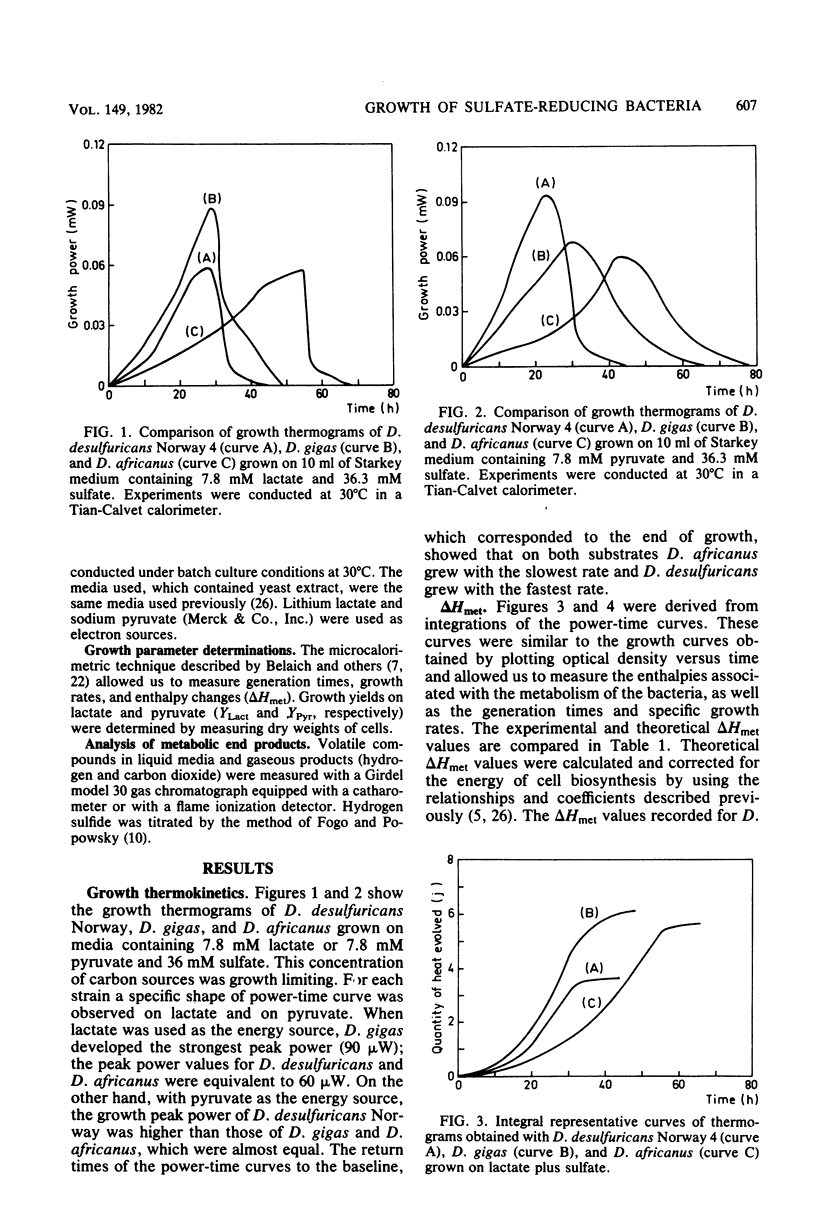
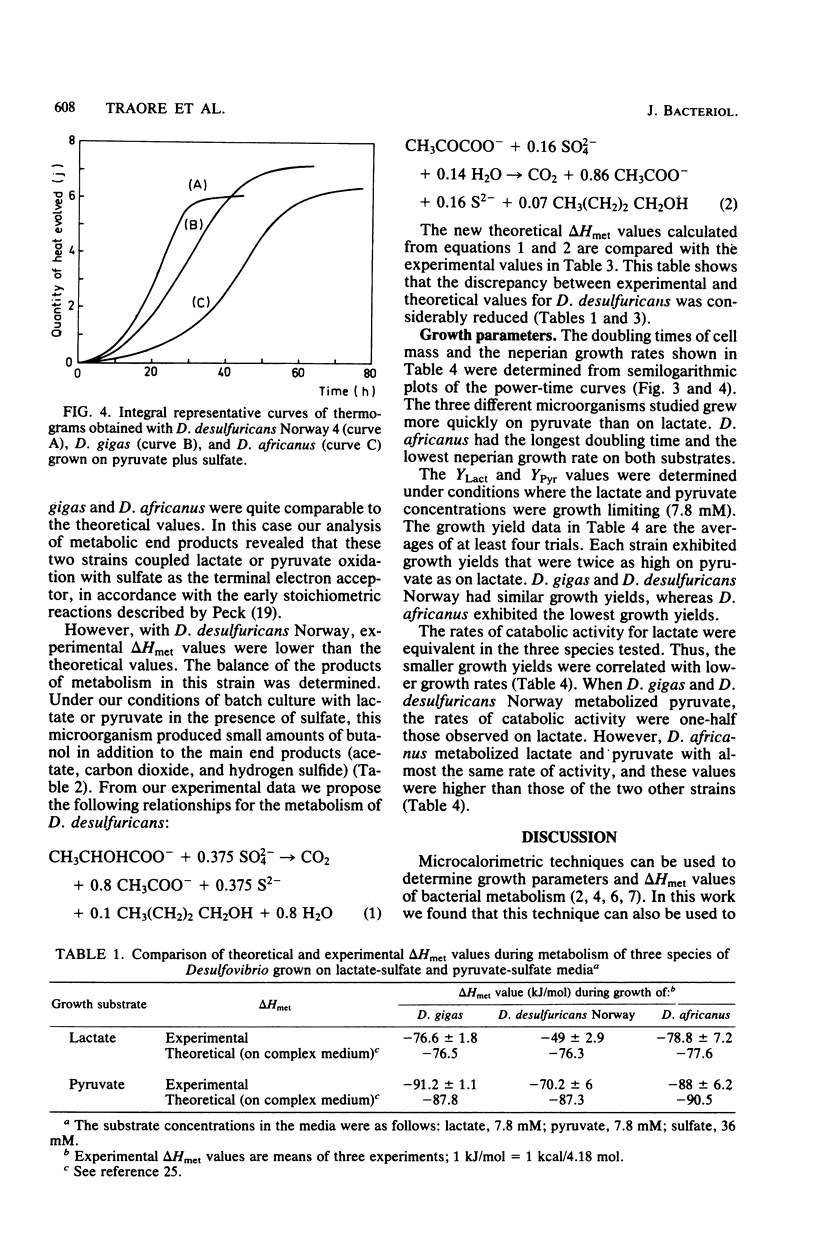
We performed a comparative study of the growth energetics of some species of Desulfovibrio by measuring microcalorimetric and molar growth yield values. Lactate and pyruvate were used as energy sources for sulfate reduction. On lactate-sulfate media Desulfovibrio desulfuricans Norway, Desulfovibrio gigas, and Desulfovibrio africanus exhibited molar growth yields of 4.1 +/- 0.6, 3.7 +/- 1.7, and 1.8 +/- 0.1 g/mol, respectively, whereas on pyruvate-sulfate media the molar growth yields were higher (8.5 +/- 0.8, 7.7 +/- 1.6, and 3.5 +/- 0.5 g/mol, respectively). Thus, we found that D. africanus was the least efficient species in converting energy into cell material. The uncoupling of energy in this strain was obvious since its catabolic activities were high compared with those of the two other strains. The enthalpy changes associated with lactate and pyruvate metabolism were -49 +/- 0.7 and -70.2 +/- 6.0 jK/mol, respectively, for D. desulfuricans, -76.6 +/- 1.8 and -91.2 +/- 1.1 kJ/mol, respectively, for D. gigas, and -78.8 +/- 7.2 and -88.0 +/- 6.2 kJ/mol, respectively, for D. africanus. D. gigas and D. africanus produced only acetate, CO2 and hydrogen sulfide as metabolic end products. In addition to these normal end products, D. desulfuricans Norway produced a small amount of butanol. This butanol production was interpreted as reflecting a regulatory system of electron flow during the catabolism of both substrates. Such metabolism was comparable to that reported for D. vulgaris, which lost part of the reducing power of its energy sources through hydrogen evolution.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- Badziong W., Thauer R. K., Zeikus J. G. Isolation and characterization of Desulfovibrio growing on hydrogen plus sulfate as the sole energy source. Arch Microbiol. 1978 Jan 23;116(1):41–49. doi: 10.1007/BF00408732. [DOI] [PubMed] [Google Scholar]
- Belaich A., Belaich J. P. Microcalorimetric study of the anaerobic growth of Escherichia coli: measurements of the affinity of whole cells for various energy substrates. J Bacteriol. 1976 Jan;125(1):19–24. doi: 10.1128/jb.125.1.19-24.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaich J. P., Senez J. C., Murgier M. Microcalorimetric study of glucose permeation in microbial cells. J Bacteriol. 1968 May;95(5):1750–1757. doi: 10.1128/jb.95.5.1750-1757.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling E. A., Blanchard G. C., Russell W. J. Bacterial identification by microcalorimetry. Nature. 1973 Feb 16;241(5390):472–473. doi: 10.1038/241472a0. [DOI] [PubMed] [Google Scholar]
- Bryant M. P., Campbell L. L., Reddy C. A., Crabill M. R. Growth of desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria. Appl Environ Microbiol. 1977 May;33(5):1162–1169. doi: 10.1128/aem.33.5.1162-1169.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W. Sulfide Production from Cysteine by Desulfovibrio desulfuricans. Appl Environ Microbiol. 1980 Feb;39(2):453–455. doi: 10.1128/aem.39.2.453-455.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Monk P. R., Wadsö I. Calorimetric identification of several strains of lactic acid bacteria. J Dairy Res. 1978 Oct;45(3):457–463. doi: 10.1017/s0022029900016678. [DOI] [PubMed] [Google Scholar]
- Laanbroek H. J., Pfennig N. Oxidation of short-chain fatty acids by sulfate-reducing bacteria in freshwater and in marine sediments. Arch Microbiol. 1981 Jan;128(3):330–335. doi: 10.1007/BF00422540. [DOI] [PubMed] [Google Scholar]
- McInerney M. J., Bryant M. P. Anaerobic Degradation of Lactate by Syntrophic Associations of Methanosarcina barkeri and Desulfovibrio Species and Effect of H(2) on Acetate Degradation. Appl Environ Microbiol. 1981 Feb;41(2):346–354. doi: 10.1128/aem.41.2.346-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk P., Wadxö I. The use of microcalorimetry for bacterial classification. J Appl Bacteriol. 1975 Feb;38(1):71–74. doi: 10.1111/j.1365-2672.1975.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Murgier M., Belaich J. P. Microcalorimetric determination of the affinity of Saccharomyces cerevisiae for some carbohydrate growth substrates. J Bacteriol. 1971 Feb;105(2):573–579. doi: 10.1128/jb.105.2.573-579.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECK H. D., Jr Symposium on metabolism of inorganic compounds. V. Comparative metabolism of inorganic sulfur compounds in microorganisms. Bacteriol Rev. 1962 Mar;26:67–94. doi: 10.1128/br.26.1.67-94.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRAT H. Observations sur la thermogenèse bactérienne. Rev Can Biol. 1953 Sep;12(1):19–34. [PubMed] [Google Scholar]
- Postgate J. R., Campbell L. L. Classification of Desulfovibrio species, the nonsporulating sulfate-reducing bacteria. Bacteriol Rev. 1966 Dec;30(4):732–738. doi: 10.1128/br.30.4.732-738.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H. A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antonie Van Leeuwenhoek. 1973;39(3):545–565. doi: 10.1007/BF02578899. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. Utilization of energy for growth and maintenance in continuous and batch cultures of microorganisms. A reevaluation of the method for the determination of ATP production by measuring molar growth yields. Biochim Biophys Acta. 1973 Feb 12;301(1):53–70. doi: 10.1016/0304-4173(73)90012-8. [DOI] [PubMed] [Google Scholar]
- Traore A. S., Hatchikian C. E., Belaich J. P., Le Gall J. Microcalorimetric studies of the growth of sulfate-reducing bacteria: energetics of Desulfovibrio vulgaris growth. J Bacteriol. 1981 Jan;145(1):191–199. doi: 10.1128/jb.145.1.191-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



