Abstract
Theories of the pathophysiology of schizophrenia have implicated the hippocampus, but controversy remains regarding hippocampal abnormalities in patients with schizophrenia. In vivo studies of hippocampal anatomy using high resolution magnetic resonance scanning and manual methods for volumetric measurement have yielded inconclusive results, perhaps because of the normal variability in hippocampal volume and the error involved in manual measurement techniques. To resolve this controversy, high dimensional transformations of a computerized brain template were used to compare hippocampal volumes and shape characteristics in 15 matched pairs of schizophrenia and control subjects. The transformations were derived from principles of general pattern matching and were constrained according to the physical properties of fluids. The analysis and comparison of hippocampal shapes based on these transformations were far superior to the comparison of hippocampal volumes or other global indices of hippocampal anatomy in showing a statistically significant difference between the two groups. In the schizophrenia subjects, hippocampal shape deformations were found to be localized to subregions of the structure that send projections to prefrontal cortex. The results of this study demonstrate that abnormalities of hippocampal anatomy occur in schizophrenia and support current hypotheses that schizophrenia involves a disturbance of hippocampal–prefrontal connections. These results also show that comparisons of neuroanatomical shapes can be more informative than volume comparisons for identifying individuals with neuropsychiatric diseases, such as schizophrenia.
The pathophysiology of schizophrenia is thought to involve abnormalities of hippocampal anatomy and function (1, 2). The hippocampus plays an important role in memory, and impairments in memory, attention, and decision-making commonly are found in schizophrenia (3). Some postmortem studies of brains of schizophrenics have suggested that the density of hippocampal pyramidal cells is decreased (4, 5); other studies have suggested hippocampal pyramidal cells are unusually small or abnormally arranged (6–9). When using manual and semi-automated methods to outline the hippocampus in high resolution magnetic resonance (MR) scans, decreases in hippocampal volumes have been reported by some (10–13), but not all (14, 15), research groups. Inconsistencies in the in vivo neuroimaging literature may exist because hippocampal volume decreases in schizophrenia are small relative to the normal variability of hippocampal volumes and the error associated with manual techniques for outlining small neuroanatomical structures (16–18). Quantitative analyses of hippocampal shape, which might be more sensitive than volumetric assessment of the structure in detecting small losses of volume in brain structure subregions, have not been carried out in subjects with neuropsychiatric diseases, such as schizophrenia.
Computerized tools for neuromorphometry, involving the high dimensional transformation of neuroanatomical templates onto sets of target MR scans, have been developing rapidly over the past decade. Detailed neuroanatomical information, such as the surface boundaries of the hippocampus, can be embedded into the template by experts and then automatically transferred to target MR images during the transformations. Because the dimensionality of the transformations is equivalent to the number of pixels in the MR scans, these methods provide a highly precise and quantitative understanding of neuroanatomical volumes and shapes, despite the variability inherent to normal anatomy (19–23). These tools have been derived from Grenander’s (24) mathematical theory of patterns, which represents the typical structures of the brain through templates and their variabilities by probabilistic transformations applied to the templates (24). We have shown previously that these tools allow for more precise estimations of hippocampal volume than manual methods for outlining the hippocampus (25, 26).
In the present study, we used transformations of a neuroanatomical template containing expert-derived information about the boundaries of the left and right hippocampus to compare subjects with schizophrenia and matched controls. An analysis of hippocampal shape as well as volume was carried out. To highlight the specificity of the shape comparison findings, hippocampal shape deformations found in the schizophrenia subjects were compared with patterns of normal hippocampal shape variability and to the hippocampal shape deformation found in a single subject with mild dementia of the Alzheimer type.
METHODS
Subject Selection and Assessment.
Fifteen subjects with schizophrenia and 15 healthy controls were recruited for participation in this study. Informed consent was obtained from all subjects after the nature and possible consequences of the study were explained. The subjects were matched in pairs with regard to gender, age, and parental socioeconomic status. The mean (SD) age for the schizophrenia subjects was 32.9 (10.4) years, and for the controls it was 30.9 (9.0) years. The Hollingshead socioeconomic status score (SD) for parents of the schizophrenia subjects was 45.4 (19.1), and for the controls it was 39.4 (17.6). Eleven subject pairs were male, and four subject pairs were female; all subjects were right-handed. The subjects with schizophrenia had been ill for a mean (SD) of 109.7 (110.9) months. All subjects were diagnosed using Diagnostic and Statistical Manual for Mental Disorders-Fourth Edition (DSM-IV) criteria (27), usually by the consensus of two diagnosticians, a research psychiatrist who had conducted a semi-structured interview, and a specially trained research assistant who had used the Structured Clinical Interview for the DSM-IV. Seven schizophrenia subjects met criteria for the undifferentiated subtype, seven for the paranoid subtype, and one for the catatonic subtype of illness. The healthy controls had never been mentally ill and had no known relatives with a psychiatric or neurologic disorder. No subject met DSM-IV criteria for either substance abuse or dependence for 3 months preceding the study. Data from five subjects with schizophrenia and five controls have been previously reported in a study to determine the reliability of hippocampal volume determinations by using high dimensional brain mapping (26). The schizophrenia subjects had been treated with antipsychotic drugs and were in partial remission from their symptoms. Residual symptoms were assessed by using the Brief Psychiatric Rating Scale (BPRS) (28). The mean (SD) total BPRS score (anchored at 1) for the schizophrenia subjects was 31.1 (5.8).
MRI and Image Preparation.
MR scans were obtained by using a Siemans Magnetom SP-4000 1.5T imaging system, a standard head coil, and a magnetization prepared rapid gradient echo (MPRAGE) sequence. The MPRAGE sequence (TR/TE, 10/4; ACQ, 1; matrix, 256 × 256; scanning time, 11.0 min) produced three-dimensional data sets with 1 × 1 mm in plane resolution and 1.25-mm slice thicknesses across the entire cranium. Sixteen-bit MR data sets were scaled and compressed to 8 bits by using global histogram equalization routines to maximize contrast at cerebrospinal fluid/gray matter interfaces. Scaling discrepancies caused by voxel anisotropy were corrected by resampling into isotropic voxels of 256 × 256 × 160. Gray scale data were normalized by using a commercially available method (Jandel Scientific, San Rafael, CA). Five Gaussian curves were used to fit each histogram, including peaks for white matter, gray matter, and cerebrospinal fluid and peaks to represent partial volume pixels resulting from white–gray and gray–cerebrospinal fluid mixtures.
In each MR scan, landmarks were placed at external brain boundaries, at points where the anterior and posterior commissures intersected the midsagittal plane, and on the surface of the hippocampus. Landmarks were placed along the surface of each hippocampus in accordance with the principal axis of the structure. Five 1-mm slices parallel to this axis were identified, and landmarks were placed at the anterior, inferior, posterior, and superior midpoints of the structure in each slice. In the most medial slice, landmarks were placed at the anterior, inferior, and superior midpoints of the pes hippocampus.
The neuroanatomical template was produced by using an MR image from a separate healthy control, and the left and right hippocampi were outlined manually by the consensus of three experts (J.H., L.W., and M.G.) by using neuroanatomical boundaries previously described by our group and others (10, 15, 26). Anatomical details of the template that were obviously anomalous were removed. The same landmarks were placed in the neuroanatomical template as had been placed in each of the target scans.
High Dimensional Brain Mapping.
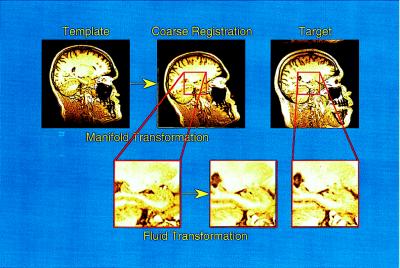
Transformation of the template onto the 30 target MR scans occurred in a two-step process (see Fig. 1). The template was first coarsely aligned to each target scan by the previously placed landmarks, and then the local anatomy was defined by a fluid transformation (20). Vector displacements of the voxels in the template during the fluid transformations were constrained by assuming that the three-dimensional surfaces and other anatomical features of the template had the physical properties of a fluid. The continuum mechanics-based mathematical derivations that underlie these transformations are reported elsewhere (19, 20–23).
Figure 1.
High dimensional transformations proceeded in a two-step process. The template was produced by using a MR scan from a healthy control subject, and information about the boundaries of the hippocampus was placed within this template by experts by using manual outlining methods. Landmarks to indicate approximate brain and hippocampal boundaries were placed in the template and each of the target scans. The first step in the transformation was guided by these landmarks and roughly oriented the template to the target scans. The second step of the transformation, performed in blocks of tissue containing the left and right hippocampus, was driven by individual voxel gray scale values but was constrained by fluid physical properties.
To determine hippocampal shape as well as volume characteristics, a triangulated graph of points was superimposed onto the surface of the hippocampus in the template and then carried along as the template was transformed onto the target scans. Computing the transformation vector fields from this graphical surface generated mathematically optimal representations of the hippocampus in the schizophrenia and control groups. Left and right hippocampal volumes in each target then were estimated by calculating the volumes enclosed by the transformed hippocampal surfaces. A pooled, within group, covariance matrix then was computed from the transformation vector fields to compare the shape characteristics of the hippocampus in the two subject groups. This covariance matrix was reduced in its dimensionality by computing the complete orthonormal set of eigenvectors that were specific to the shape of the hippocampus (24).
Total brain volumes were derived from elastic-based transformations (i.e., eight basis vectors and 2,187 coefficients), so that comparisons of hippocampal volumes could be examined before and after hippocampal volumes had been covaried for total brain volumes.
Data Analysis.
A two-way, repeated measures ANOVA, with diagnostic group and hemisphere as factors, was used to compare hippocampal volumes in the schizophrenia and control subjects. The first 15 eigenvectors were chosen a priori as adequately representing hippocampal shapes, and a linear discriminant function was computed by using the vectors with the largest eigenvectors. Based on jack-knifed classification rates, a linear combination of the first six eigenvectors in sequence provided a statistically significant classification of the subjects. However, a more optimal solution was obtained based on a stepwise procedure and by using the first, third, fourth, sixth, tenth, and fifteenth eigenvectors. Log likelihood ratio values were calculated as a measure of hippocampal shape in each subject according to both solutions, and the statistical significance of group differences was tested by using Wilk’s Lambda.
Displacements of the hippocampal surface that discriminated the two groups were visualized as maps of simple differences. In addition, maps were constructed of z-scores values at every point of the graphical surface of the left and right hippocampi. The z-scores were calculated as the square root of the quotient of the difference between the two group vectors in three dimensions and the inverse of the covariance matrix multiplied by the difference of the two group vectors.
RESULTS
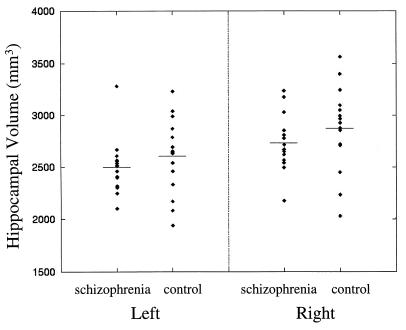
Fig. 2 illustrates the comparison of hippocampal volumes in the schizophrenia and control subjects derived from the transformations. The mean (SD) hippocampal volume for the schizophrenia subjects was 2,497 (264) mm3 in the left hemisphere and 2,730 (270) mm3 in the right hemisphere, and the mean (SD) hippocampal volume for the control subjects was 2,603 (364) mm3 in the left hemisphere and 2,874 (410) mm3 in the right hemisphere. However, hippocampal volume estimates, with or without being adjusted for the subject’s total brain volume, failed to discriminate the two groups of subjects. A comparison of left and right hippocampal volumes not covaried for total brain volume did not result in a statistically significant group difference (F = 1.25, df = 1, 28, P = 0.27). Similarly, a comparison of left hippocampal volumes covaried for total brain volumes (F = 1.12, df = 1, 28, P = 0.30), and a comparison of right hippocampal volumes covaried for total brain volumes (F = 1.84, df = 1, 28, P = 0.19) did not result in statistically significant group differences. Furthermore, a comparison of the two subject groups with regard to the global scale and skew of the hippocampus in three dimensions as derived from the transformations gave a similarly weak result (Hotelling’s t2 = 11.8, df = 6, P = 0.19). Left and right hippocampal volumes were significantly different in the combined group of subjects (F = 38.52, df = 1, 28, P = 0.0001), the left hippocampal volume being ≈10% smaller than the right. There were no statistically significant interactions between diagnostic group and brain hemisphere.
Figure 2.
Hippocampal volumes were estimated by calculating the voxels enclosed by hippocampal surfaces that had been carried along through the high dimensional transformations. A comparison of the volumes in the two groups was not statistically significant (see text).
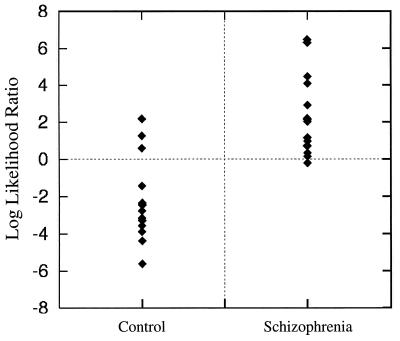
A comparison of hippocampal shape characteristics in the schizophrenia and control subjects using the eigenvector values from reducing the dimensionality of the covariance matrix is shown in Fig. 3. Log likelihood ratio values, calculated as the linear combination of five eigenvectors derived from a stepwise procedure (i.e., eigenvectors 1, 3, 4, 6, 10, and 15), strongly discriminated the two groups of subjects (F = 4.726, df = 1, 28, P = 0.0028). Also, using this combination of eigenvectors, 12 of 15 subjects in both groups could be classified correctly in a jack-knife analysis in which each subject being assessed was removed in turn from the calculation before generating the statistical model. Use of the first six eigenvectors in sequence also showed a statistically significant, but somewhat smaller, difference between the two groups (F = 2.68, df = 1, 28, P = 0.040). To further test the robustness of the shape comparison, a distribution free estimate of the level of significance was carried out to compare the two groups by using the first four eigenvectors (29). This statistical test also indicated a statistically significant difference between the two groups (P = .023).
Figure 3.
A comparison of individual log likelihood ratio values in the two groups showed a strong statistically significant difference (see text). The values shown were derived from the optimal linear combination of eigenvectors (i.e., 1, 3, 4, 6, 10, and 15), determined by a stepwise process. When we used this method of comparison, 12 of 15 subjects in each group were classified correctly.
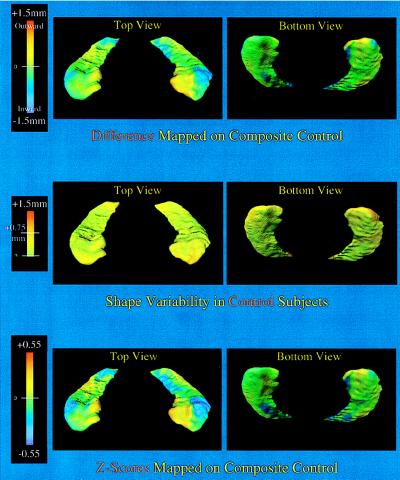
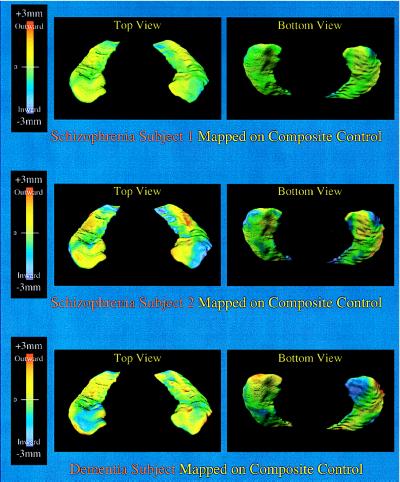
Hippocampal surface deformations that formed the basis for the statistically significant shape difference between the schizophrenia subjects and the controls are shown in Fig. 4. These deformations, shown as either simple surface displacements or z-scores, were similar on each side and involved specific subregions of the head and body of the hippocampus. As shown in Fig. 4, the pattern of hippocampal shape variability in the control subjects was not related to the distribution of disease-related deformations. Rather, normative hippocampal shape variation was evenly distributed on the hippocampal surface and was of relatively small magnitude compared with the deformations associated with schizophrenia. Fig. 5 contrasts the pattern of shape deformations found in two individual subjects with schizophrenia with the pattern of shape deformation found in a single subject with mild dementia of the Alzheimer type. The pattern of hippocampal shape deformity in the schizophrenia subjects was distinct from both the pattern of normal variability and the pattern of deformity found in the subject with dementia of the Alzheimer type, so our data suggest that the hippocampal shape deformities observed in subjects with schizophrenia may be relatively specific to that disorder.
Figure 4.
The hippocampal shapes shown represent the composite hippocampal surfaces in the healthy controls. (Top) The degree of displacement of these surfaces (in millimeters) perpendicular to the plane of the structure and relative to the control composite is indicated by a flame scale. The lateral aspect of the head of the hippocampus and the medial aspect of the body, where the subiculum is found, showed localized shape deformities in the schizophrenia subjects. (Middle) The pattern of shape variability in controls is shown [SD at each surface point calculated by using the optimal linear combination of six eigenfuncions (i.e., 1, 3, 4, 6, 10, 15). More than 95% of surface points had a SD <0.75 mm. (Bottom) A map of z-score values is shown to examine the shape deformities in the schizophrenia subjects while accounting for general variability in hippocampal shape. In contrast to the normal pattern of shape variability, shape deformities found in the schizophrenia subjects were highly localized.
Figure 5.
(Top and Middle) Hippocampal shape deformities in two individuals with schizophrenia with the largest log likelihood ratio values (see Fig. 3) are shown and can be compared with those found in an individual with mild dementia of the Alzheimer type and whose hippocampal volumes were similar to the schizophrenia subjects (Bottom, left hippocampal volume = 2,581 mm3, right hippocampal volume = 2448 mm3). The displacement of these surfaces (in millimeters) perpendicular to the plane of the structure and relative to the control composite is indicated by a flame scale. Shape deformities in the dementia subject were distributed differently than those found in the two schizophrenia subjects and did not specifically involve the lateral aspect of the head of the hippocampus.
Log likelihood ratio values, calculated by using the combination of six eigenvectors derived from the stepwise procedure (i.e., 1, 3, 4, 6, 10, and 15) were not correlated with left and right hippocampal volumes in the combined group of schizophrenia or in the control subjects. In the schizophrenia subjects, log likelihood ratio values also were not correlated with indicators of the clinical state or chronicity, such as total Brief Psychiatry Rating Scale (BPRS) scores and duration of illness. There were also no statistically significant correlations between hippocampal volumes and age in the combined group of subjects or between hippocampal volumes and duration of illness in the schizophrenia subjects. However, the severity of psychopathology, as assessed by total BPRS scores, was correlated with total brain volume in the schizophrenia subjects (r = 0.54, P = 0.036). In the combined group of schizophrenia and control subjects, left (r = 0.59, P = 0.001) and right (r = 0.59, P = 0.0006) hippocampal volumes were similarly correlated with total brain volume.
DISCUSSION
The results of this study indicate that there are only minimal differences (4–5%) in the volumes of the left and right hippocampi between schizophrenia and control subjects, whether or not these volumes were corrected for total brain volume. These findings are highly consistent with the results of previous individual studies and with a recent metaanalysis (30) suggesting that interindividual variability in hippocampal volumes is greater than any differences attributable to schizophrenia. Our results also show that the left hippocampal volume is generally smaller than the right hippocampal volume. This result adds to evidence from postmortem studies of similar left-to-right differences in the hippocampus and other temporal lobe structures (31–33).
The major finding of this study is that the high dimensional assessment of hippocampal shape was far superior to the comparison of hippocampal volumes and indices of global hippocampal orientation in discriminating the schizophrenia and control subjects. We tested the robustness of our finding by using more than one approach to selecting the eigenvectors to be included in the discriminant analysis and by performing a jack-knife analysis in which the subjects being tested had not been included in the generation of the statistical model. The substantial value of examining neuroanatomical shapes should not be surprising because local details of neuroanatomical shape may contain critical information about neural architecture in the mammalian brain not captured by the total volume or gross orientation of the structure. In addition, the degree of hippocampal shape deformity, expressed in each subject by a log likelihood ratio value derived from the linear combination of five eigenvectors, was not correlated with duration of illness or symptom severity, which suggests that the observed shape deformities may have been more related to the fundamental neurobiology of the disease than to the clinical state at the time of scanning or factors related to chronicity, such as the degree of prior drug treatment. The observed correlation between duration of illness and total brain volumes in the schizophrenia subjects does suggest, however, that other structures in the brain may be altered in relationship to chronicity.
Van Essen recently put forth a general hypothesis suggesting that the physical properties of neural tissue combined with the patterns of neural connectivity determine the shape of specific brain structures, especially those that are anisotropic, such as the hippocampus (34). Postmortem studies of the hippocampus and other medial temporal lobe structures suggest that schizophrenia may be associated with abnormalities in neural architecture and connectivity (4–9, 35). If such hypotheses are correct, abnormalities of neuroanatomical shape may be found in schizophrenia subjects even when there are minimal or no changes in volume. Thus, the analysis of brain structure shape may be a particularly sensitive indicator for the presence of schizophrenia, and other neuropsychiatric diseases, for which abnormalities of neurocircuitry have been hypothesized (1).
Fig. 4 shows that the superior and lateral aspects of the hippocampal head were deformed on both the left and right sides in the subjects with schizophrenia. This specific observation has important implications for hypotheses of abnormal neurocircuitry in schizophrenia. Hippocampal CA1 neurons that send projections to the medial prefrontal cortex are predominantly found in the head subregion of the hippocampus (36, 37). Therefore, our discovery of a specific deformity in this area provides important support for the hypothesis that schizophrenia involves a disturbance of the connections between medial temporal and prefrontal cortical structures (1, 37–39).
The methods used to make these assessments of hippocampal shape and volume are an extension of a large body of work on digital electronic brain atlases. These atlases have been useful for coregistration of complementary digital data sets, such as PET/SPECT, CT, and MRI (40–42). Some also can facilitate neuromorphometric analyses of complex human brain diseases (43–47). However, the characterization of neuroanatomical shape aberrations, such as those likely to occur in schizophrenia, requires the quantification of local variability in brain structure and makes the high dimensionality of the transformations essential. Exploiting important geometric features, such as point landmarks and contours, may enhance lower dimensional transformations (48–50). Our approach, while incorporating such enhancements, is fundamentally based on individual voxel data and so is more akin to the volume mapping of Bajcsy and colleagues (51).
High dimensional brain mapping is a major step forward in neuromorphometry and ultimately may lead to new tools for the diagnosis of neuropsychiatric diseases, such as schizophrenia. Currently, we lack laboratory tests to use in concert with clinical and cognitive assessments to aid in the diagnosis of such diseases. Such tests may emerge from high dimensional assessments of neuroanatomical structures. The ability to ascertain diagnosis when symptoms are minimal and of brief duration would allow for earlier treatment and perhaps would prevent some of the disability now associated with many neuropsychiatric diseases. In addition, high dimensional brain mapping should allow us to develop and test more sophisticated hypotheses of the pathophysiology of neuropsychiatric diseases within a precise neuroanatomical framework.
Acknowledgments
We thank April Ratanasadudi for her assistance with subject recruitment and assessment. Fred Bookstein made helpful suggestions regarding our methods for shape analysis, and David Van Essen, Joel Price, and Robert McCarley made helpful comments on the manuscript. This work was supported by the Army Center for Imaging Science, National Institutes of Health Grants NS 34050, NS 35368, MH 525158, and MH 56584, National Science Foundation Grant BIR-9 424264, and the Gregory B. Couch Endowment at Washington University.
References
- 1.Csernansky J G, Murphy G M, Faustman W O. Biol Psychiatry. 1991;30:383–400. doi: 10.1016/0006-3223(91)90295-w. [DOI] [PubMed] [Google Scholar]
- 2.Roberts G W. Trends Neurosci. 1990;13:207–211. doi: 10.1016/0166-2236(90)90161-3. [DOI] [PubMed] [Google Scholar]
- 3.Saykin A J, Shtasel D L, Gur R E, Kester D B, Mozley L H, Stafiniak P, Gur R C. Arch Gen Psych. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 4.Falkai P, Bogerts B. Eur Arch Psychiatr Neurol Sci. 1986;236:154–161. doi: 10.1007/BF00380943. [DOI] [PubMed] [Google Scholar]
- 5.Jeste D V, Lohr J B. Arch Gen Psychiatry. 1989;46:1019–1024. doi: 10.1001/archpsyc.1989.01810110061009. [DOI] [PubMed] [Google Scholar]
- 6.Benes F M, Sorensen I, Bird E D. Schizophrenia Bull. 1991;17:597–608. doi: 10.1093/schbul/17.4.597. [DOI] [PubMed] [Google Scholar]
- 7.Arnold SE, Franz B R, Gur R C, Gur R E, Shapiro R M, Moberg P J, Trojanowski J Q. Am J Psychiatry. 1995;152:738–748. doi: 10.1176/ajp.152.5.738. [DOI] [PubMed] [Google Scholar]
- 8.Conrad A J, Abebe T, Austin R, Forsythe S, Scheibel A B. Arch Gen Psychiatry. 1991;48:413–417. doi: 10.1001/archpsyc.1991.01810290025003. [DOI] [PubMed] [Google Scholar]
- 9.Zaidel D W, Esiri M M, Harrison P J. Am J Psychiatry. 1997;154:812–818. doi: 10.1176/ajp.154.6.812. [DOI] [PubMed] [Google Scholar]
- 10.Shenton M E, Kikinis R, Jolesz F A, Pollak S D, LeMay M, Wible C G, Hokama H, Martin J, Metcalf D, Coleman M, McCarley R W. N Engl J Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- 11.Bogerts B, Lieberman J A, Ashtari M, Bilder R M, Degreef G, Lerner G, John C, Masiar S. Biol Psychiatry. 1993;33:236–246. doi: 10.1016/0006-3223(93)90289-p. [DOI] [PubMed] [Google Scholar]
- 12.Breier A, Buchanan R W, Elkashef A, Munson R C, Kirkpatrick B, Gellad F. Arch Gen Psychiatry. 1992;49:921–926. doi: 10.1001/archpsyc.1992.01820120009003. [DOI] [PubMed] [Google Scholar]
- 13.Rossi A, Stratta P, Mancini F, Gallucci M, Mattei P, Core L, DiMichele V, Casacchia M. Psychiatry Res. 1994;52:43–53. doi: 10.1016/0165-1781(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 14.Swayze V W, II, Andreasen N C, Alliger R J, Yuh W T C, Ehrhardt J C. Biol Psychiatry. 1992;31:221–240. doi: 10.1016/0006-3223(92)90046-3. [DOI] [PubMed] [Google Scholar]
- 15.Zipursky R B, Marsh L, Lim K O, DeMent S, Shear P K, Sullivan E V, Murphy G M, Csernansky J G, Pfefferbaum A. Biol Psychiatry. 1994;35:501–516. doi: 10.1016/0006-3223(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 16.Jack C R, Twomey C K, Zinsmeister A R, Sharbrough F W, Peterson R C, Cascino G D. Radiology. 1989;172:549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- 17.Bartzokis G, Mintz J, Marx P, Osborn D, Gutkind D, Chiang F, Phelan C K, Marder S R. Magn Reson Imaging. 1993;11:993–1006. doi: 10.1016/0730-725x(93)90218-3. [DOI] [PubMed] [Google Scholar]
- 18.Pearlson G D, Marsh L. In: Annual Review of Psychiatry. Oldham J, Riba M B, Tasman A, editors. Vol. 12. Washington, DC: Am. Psych. Assoc.; 1993. pp. 347–382. [Google Scholar]
- 19.Miller M I, Christensen G E, Amit Y, Grenander U. Proc Natl Acad Sci. 1993;90:11944–11948. doi: 10.1073/pnas.90.24.11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chistensen G E, Rabbitt R D, Miller M I. Physics Med Biol. 1994;39:609–618. doi: 10.1088/0031-9155/39/3/022. [DOI] [PubMed] [Google Scholar]
- 21.Grenander U, Miller M I. J R Stat Soc Bull. 1994;56:549–603. [Google Scholar]
- 22.Christensen G E, Rabbitt R D, Miller M I. IEEE Trans Image Process. 1996;5:1–13. doi: 10.1109/83.536892. [DOI] [PubMed] [Google Scholar]
- 23.Miller M I, Banerjee A, Christensen G E, Joshi S C, Khaneja N, Grenander U, Matejic L. Stat Methods Med Res. 1997;6:267–299. doi: 10.1177/096228029700600305. [DOI] [PubMed] [Google Scholar]
- 24.Grenander U. General Pattern Theory. London: Oxford Univ. Press; 1991. [Google Scholar]
- 25.Haller J W, Christensen G E, Joshi S C, Newcomer J W, Miller M I, Csernansky J G, Vannier M W. Radiology. 1996;199:787–792. doi: 10.1148/radiology.199.3.8638006. [DOI] [PubMed] [Google Scholar]
- 26.Haller J W, Banerjee A, Christensen G E, Gado M, Joshi S, Miller M I, Sheline Y I, Vannier M W, Csernansky J G. Radiology. 1997;202:504–510. doi: 10.1148/radiology.202.2.9015081. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) Washington, DC: Am. Psych. Assoc.; 1994. [Google Scholar]
- 28.Overall J E. In: Psychological Measurements in Psychopharmacology: Modern Problems in Pharmacopsychiatry. Pichot P, editor. Vol. 7. Basel: Karger; 1974. pp. 67–78. [PubMed] [Google Scholar]
- 29.Bradley J V. Distribution-Free Statistical Methods. New York: Prentice-Hall; 1968. [Google Scholar]
- 30.Nelson M D, Saykin A J, Flashman L A, Riordan H J. Arch Gen Psychiatry. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- 31.Geschwind N, Letvitsky W. Science. 1968;161:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- 32.Galaburda A M, Corsiglia J, Rosen G D, Sherman G F. Neuropsychologia. 1987;25:853–868. [Google Scholar]
- 33.Galaburda A M. Biological Asymmetry and Handedness (Ciba Foundation Symposium 162) Chichester, U.K.: Wiley; 1991. pp. 219–233. [Google Scholar]
- 34.Van Essen D. Nature (London) 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- 35.Arnold SE, Hyman B T, Van Hoesen G W, Damasio A R. Arch Gen Psychiatry. 1991;48:625–632. doi: 10.1001/archpsyc.1991.01810310043008. [DOI] [PubMed] [Google Scholar]
- 36.Barbas H, Blatt G J. Hippocampus. 1995;5:511–533. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- 37.Carmichael S T, Price J L. J Comp Neurology. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 38.Barbas H. Neurosci Biobehav Rev. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- 39.Weinberger D R, Berman K F, Suddath R, Torrey E F. Am J Psychiatry. 1992;149:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- 40.Pechura C M, Martin J B. Mapping the Brain and its Functions: Integrating Enabling Technologies in Neuroscience Research. Washington, DC: Institute of Medicine; 1991. [PubMed] [Google Scholar]
- 41.Greitz T, Bohm C, Holte S, Eriksson L. J Comp Assist Tomogr. 1991;15:26–28. [PubMed] [Google Scholar]
- 42.Dann R, Hoford J, Kovacic S, Reivich M, Bajscy R. J Comp Assist Tomogr. 1989;13:603–611. doi: 10.1097/00004728-198907000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Hohne K H, Bomans M, Reimer M, Schubert R, Tiede U, Lierse W. IEEE Comput Graphics Appl. 1992;12:72–78. [Google Scholar]
- 44.Jansen W, Baak J P, Smeulder A W, van Ginneken A M. Pathol Res Pract. 1989;185:652–656. doi: 10.1016/S0344-0338(89)80211-0. [DOI] [PubMed] [Google Scholar]
- 45.Bohm C, Greitz T, Berggren B, Ollson L. Am J Neuroradiol Res. 1988;4:731–733. [PMC free article] [PubMed] [Google Scholar]
- 46.Evans A C, Beil C, Marret S, Thompson C J, Hakim J. J Cerebral Blood Flow Metabol. 1988;8:513–530. doi: 10.1038/jcbfm.1988.92. [DOI] [PubMed] [Google Scholar]
- 47.Evans A C, Dai W, Collins L, Neelin P, Marret S. Image Processing. 1991;145:236–246. [Google Scholar]
- 48.Bookstein F L. The Measurement of Biological Shape and Shape Change. Vol. 24. New York: Springer-Verlag: Lecture Notes in Biomathematics; 1978. [Google Scholar]
- 49.Bookstein F L, Green W D K. In: Visualization in Biomedical Computing. Robb R A, editor. Washington, DC: SPIE; 1992. pp. 242–258. [Google Scholar]
- 50.Toga A W, Banerjee P K, Payne B A. J Cereb Blood Flow Metabol. 1991;11:S560. [Google Scholar]
- 51.Bajcsy R, Lieberson R, Reivich M. J Comp Assist Tomogr. 1983;7:618–625. doi: 10.1097/00004728-198308000-00008. [DOI] [PubMed] [Google Scholar]