Abstract
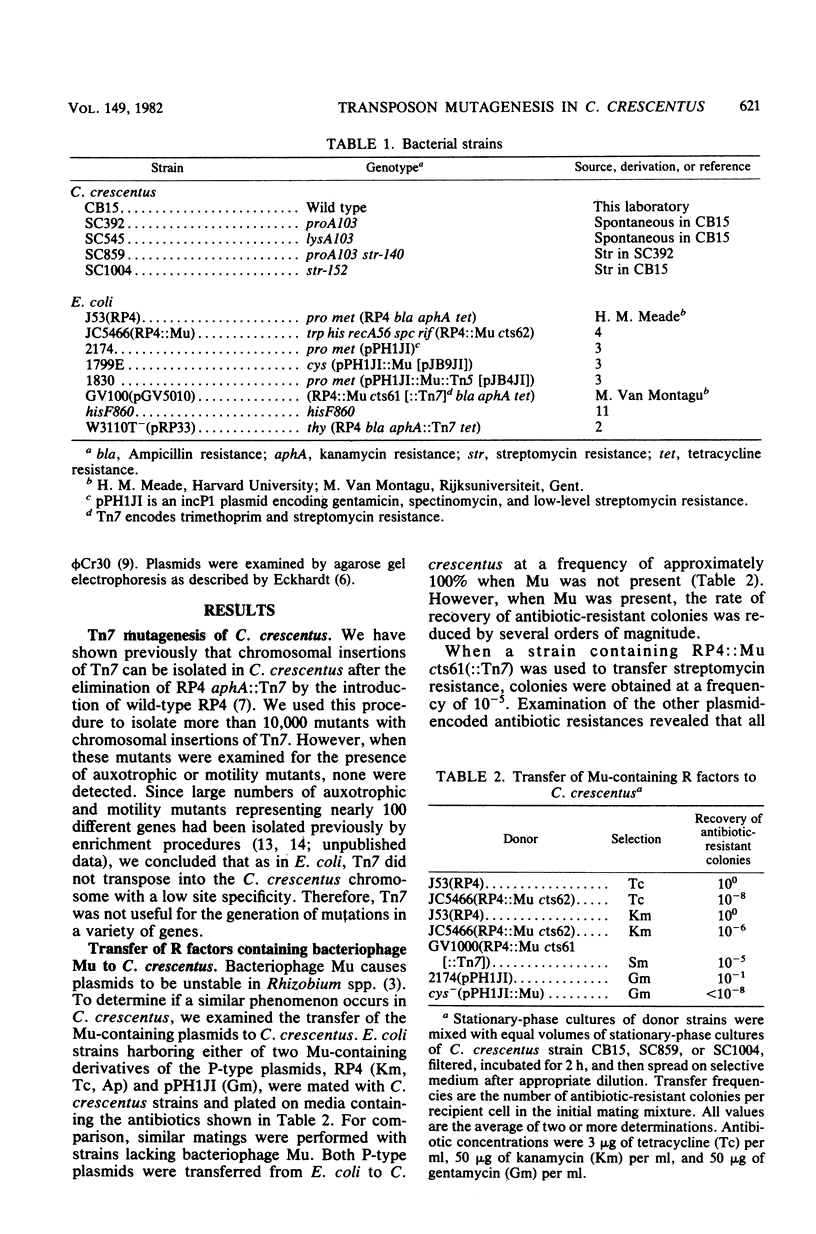
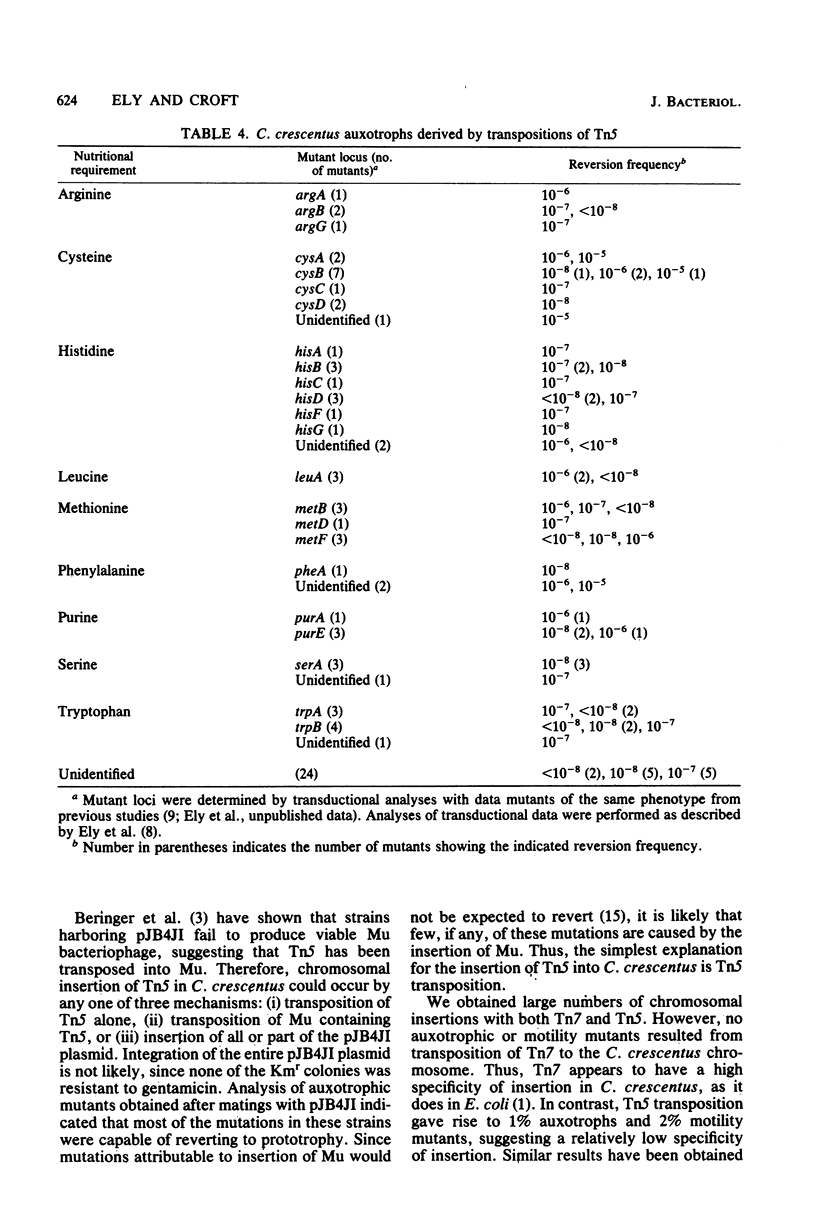
Transposons Tn5 (Km) and Tn7 (Tp and Sm) were transferred to Caulobacter crescentus via P-type antibiotic resistance factors. Transposition was demonstrated by the isolation of chromosomal insertions of each transposon. With C. crescentus strains harboring RP4 aphA::Tn7, the introduction of a wild-type RP4 resulted in the loss of the resident plasmid. Simultaneous selection for Kmr and Smr yielded colonies with chromosomal insertions of Tn7. Examination of over 10,000 chromosomal insertions of Tn7 indicated no auxotrophic or motility mutants. Thus, Tn7 appears to have a high specificity of insertion in C. crescentus. The Mu-containing plasmid pJB4JI transferred Tn5 to C. crescentus, but the plasmid was not maintained. Control experiments showed that recovery of Mu-containing plasmids occurred at very low frequencies in C. crescentus and that the plasmids which were recovered had undergone extensive deletion of plasmid DNA. Presumably, some part of the Mu genome was not tolerated by C. crescentus. The instability of the Mu-containing plasmids makes them excellent vectors for the introduction of transposons, and we have used pJB4JI to isolated chromosomal insertions of Tn5. When several thousand of these insertion mutants were examined, we found auxotrophic and motility mutants at frequencies of 1 and 2%, respectively. These results indicate that Tn5 had a low specificity of insertion in C. crescentus and therefore would be a useful mutagen for obtaining a variety of mutant phenotypes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth P. T., Datta N., Hedges R. W., Grinter N. J. Transposition of a deoxyribonucleic acid sequence encoding trimethoprim and streptomycin resistances from R483 to other replicons. J Bacteriol. 1976 Mar;125(3):800–810. doi: 10.1128/jb.125.3.800-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth P. T., Grinter N. J. Map of plasmid RP4 derived by insertion of transposon C. J Mol Biol. 1977 Jul 5;113(3):455–474. doi: 10.1016/0022-2836(77)90233-9. [DOI] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Ely B., Amarasinghe A. B., Bender R. A. Ammonia assimilation and glutamate formation in Caulobacter crescentus. J Bacteriol. 1978 Jan;133(1):225–230. doi: 10.1128/jb.133.1.225-230.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B., Johnson R. C. Generalized Transduction in CAULOBACTER CRESCENTUS. Genetics. 1977 Nov;87(3):391–399. doi: 10.1093/genetics/87.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B. Transfer of drug resistance factors to the dimorphic bacterium Caulobacter crescentus. Genetics. 1979 Mar;91(3):371–380. doi: 10.1093/genetics/91.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel D. J., Nester E. W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol. 1980 Nov;144(2):732–743. doi: 10.1128/jb.144.2.732-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick-Silversmith L., Hartman P. E. Histidine-requiring mutants of Escherichia coli K12. Genetics. 1970 Oct;66(2):231–244. doi: 10.1093/genetics/66.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshey R. M., Bukhari A. I. A mechanism of DNA transposition. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1090–1094. doi: 10.1073/pnas.78.2.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Ely B. Analysis of nonmotile mutants of the dimorphic bacterium Caulobacter crescentus. J Bacteriol. 1979 Jan;137(1):627–634. doi: 10.1128/jb.137.1.627-634.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Ely B. Isolation of spontaneously derived mutants of Caulobacter crescentus. Genetics. 1977 May;86(1):25–32. doi: 10.1093/genetics/86.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan E., Saedler H., Starlinger P. O0 and strong-polar mutations in the gal operon are insertions. Mol Gen Genet. 1968;102(4):353–363. doi: 10.1007/BF00433726. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Kuner J. M., Kaiser D. Introduction of transposon Tn5 into Myxococcus for analysis of developmental and other nonselectable mutants. Proc Natl Acad Sci U S A. 1981 Jan;78(1):425–429. doi: 10.1073/pnas.78.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K. J., Berg C. M. Escherichia coli K-12 auxotrophs induced by insertion of the transposable element Tn5. Genetics. 1979 Jul;92(3):741–747. doi: 10.1093/genetics/92.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet F., Silva B., van Montagu M., Schell J. Transfer of RP4::mu plasmids to Agrobacterium tumefaciens. Plasmid. 1978 Sep;1(4):446–455. doi: 10.1016/0147-619x(78)90003-3. [DOI] [PubMed] [Google Scholar]



