Abstract
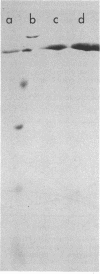
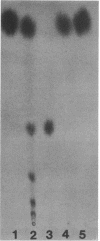
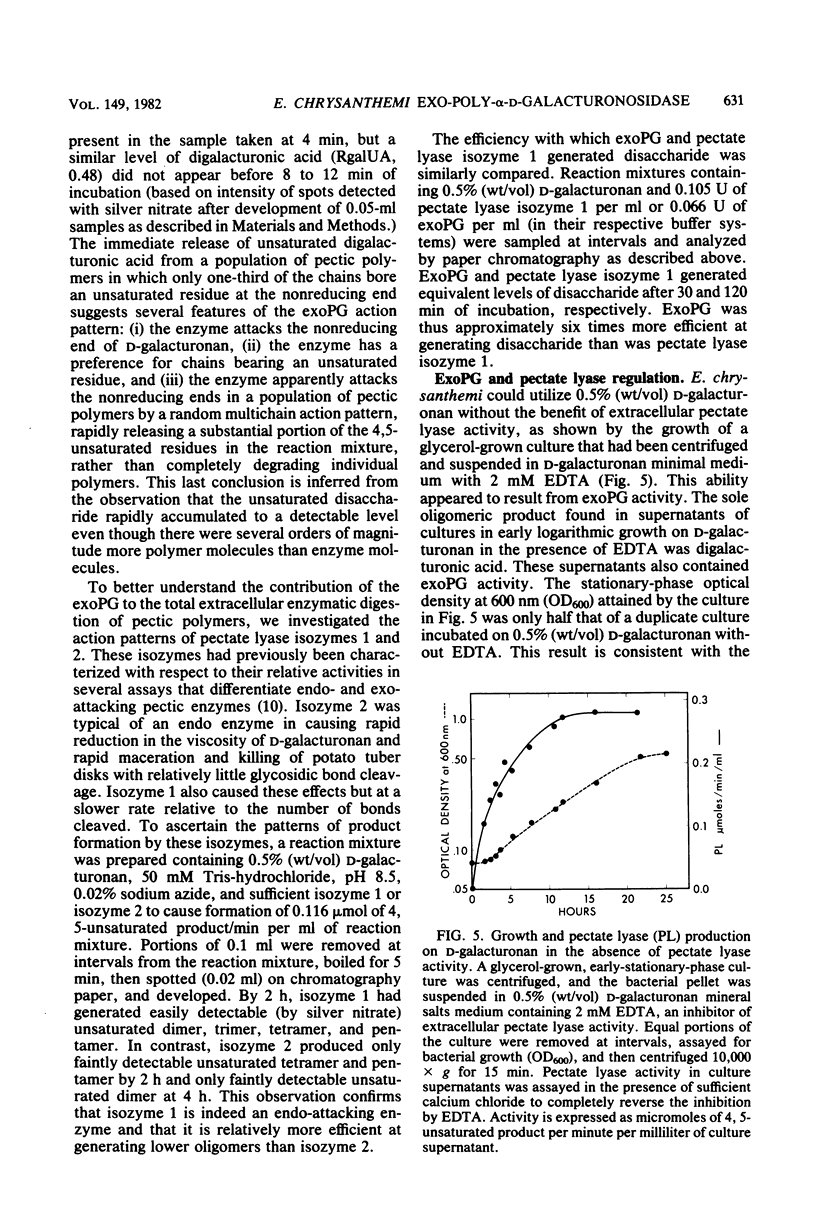
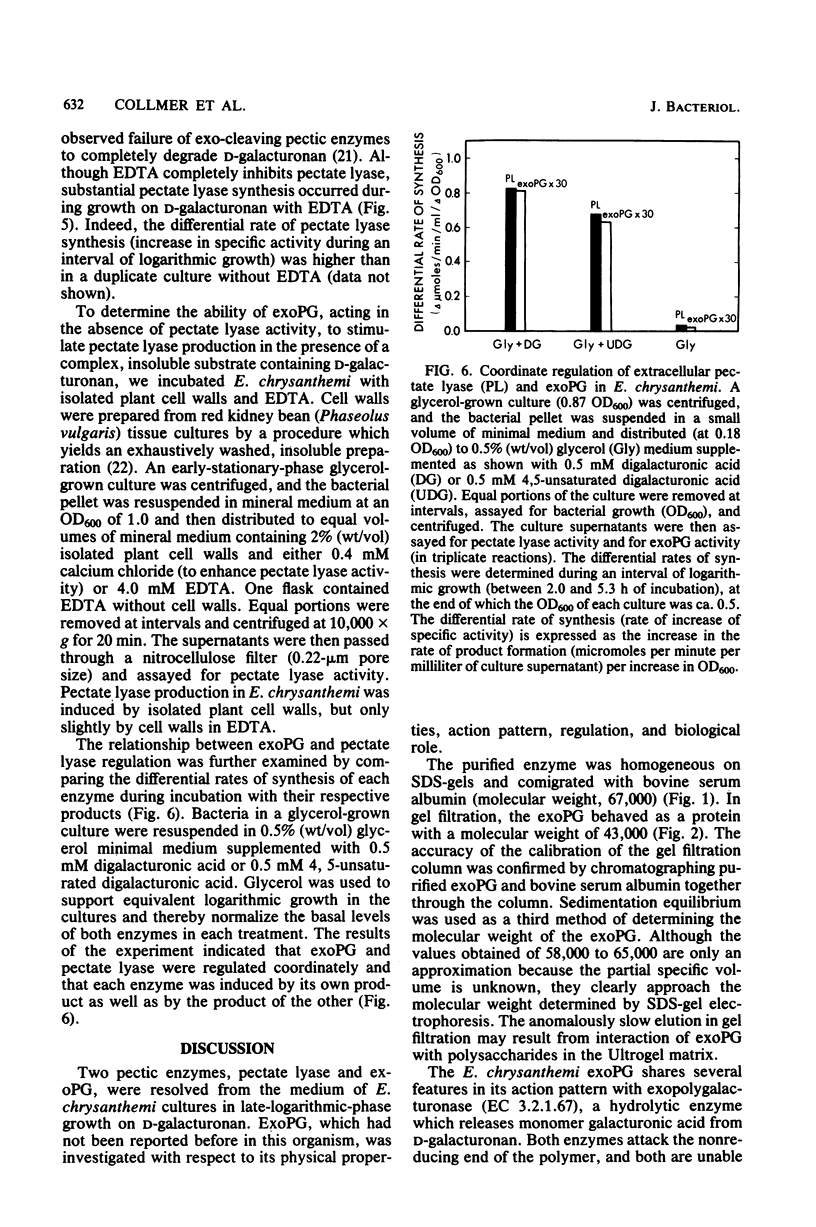
Pectic enzymes in the supernatants of Erwinia chrysanthemi cultures in late-logarithmic-phase growth on D-galacturonan were resolved into three components: two pectate lyase isozymes and an exo-poly-alpha-D-galacturonosidase previously unreported in this organism. The hydrolytic enzyme was purified to homogeneity by ammonium sulfate fractionation, preparative electrofocusing in Ultrodex gel, and gel filtration through Ultrogel AcA54. The enzyme had a specific activity of 591 mumol/min per mg of protein, a pI of 8.3, a molecular weight of 67,000, a pH optimum of 6.0, and a Km of 0.05 mM for D-galacturonan. Analyses of reaction mixtures by paper chromatography revealed that the enzyme released only digalacturonic acid from D-galacturonan. The action of the hydrolytic enzyme on D-galacturonan labeled at the nonreducing end by partial digestion with pectate lyase revealed that it rapidly released 4,5-unsaturated digalacturonic acid from 4,5-unsaturated pectic polymers. The production of extracellular exo-poly-alpha-D-galacturonosidase was coordinately regulated with pectate lyase production. The action patterns of the two enzymes appeared complementary in the degradation of pectic polymers to disaccharides that stimulated pectic enzyme production and supported bacterial growth.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpita N., Sabularse D., Montezinos D., Delmer D. P. Determination of the pore size of cell walls of living plant cells. Science. 1979 Sep 14;205(4411):1144–1147. doi: 10.1126/science.205.4411.1144. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Donor strains of the soft-rot bacterium Erwinia chrysanthemi and conjugational transfer of the pectolytic capacity. J Bacteriol. 1977 Dec;132(3):862–869. doi: 10.1128/jb.132.3.862-869.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collmer A., Bateman D. F. Impaired induction and self-catabolite repression of extracellular pectate lyase in Erwinia chrysanthemi mutants deficient in oligogalacturonide lyase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3920–3924. doi: 10.1073/pnas.78.6.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepeau R. H., Hensley C. P., Jr, Edelstein S. J. Analytical ultracentrifugation with absorption optics and a scanner-computer system. Applications to molecular weight measurements on interacting systems. Biochemistry. 1974 Nov 5;13(23):4860–4865. doi: 10.1021/bi00720a027. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACMILLAN J. D., PHAFF H. J., VAUGHN R. H. THE PATTERN OF ACTION OF AN EXOPOLYGALACTURONIC ACID-TRANS-ELIMINASE FROM CLOSTRIDIUM MULTIFERMENTANS. Biochemistry. 1964 Apr;3:572–578. doi: 10.1021/bi00892a017. [DOI] [PubMed] [Google Scholar]
- Nasuno S., Starr M. P. Polygalacturonase of Erwinia carotovora. J Biol Chem. 1966 Nov 25;241(22):5298–5306. [PubMed] [Google Scholar]
- Rexová-Benková L., Markovic O. Pectic enzymes. Adv Carbohydr Chem Biochem. 1976;33:323–385. doi: 10.1016/s0065-2318(08)60285-1. [DOI] [PubMed] [Google Scholar]
- Rexová-Benková L. The size of the substrate-binding site of an Aspergillus niger extracellular endopolygalacturonase. Eur J Biochem. 1973 Nov 1;39(1):109–115. doi: 10.1111/j.1432-1033.1973.tb03109.x. [DOI] [PubMed] [Google Scholar]
- Talmadge K. W., Keegstra K., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: I. The Macromolecular Components of the Walls of Suspension-cultured Sycamore Cells with a Detailed Analysis of the Pectic Polysaccharides. Plant Physiol. 1973 Jan;51(1):158–173. doi: 10.1104/pp.51.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuyumu S. "Self-catabolite repression" of pectate lyase in Erwinia carotovora. J Bacteriol. 1979 Feb;137(2):1035–1036. doi: 10.1128/jb.137.2.1035-1036.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuyumu S. Inducer of pectic acid lyase in Erwinia carotovora. Nature. 1977 Sep 15;269(5625):237–238. doi: 10.1038/269237a0. [DOI] [PubMed] [Google Scholar]
- WARREN L. Thiobarbituric acid spray reaction for deoxy sugars and sialic acids. Nature. 1960 Apr 16;186:237–237. doi: 10.1038/186237a0. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Zucker M., Hankin L. Regulation of pectate lyase synthesis in Pseudomonas fluorescens and Erwinia carotovora. J Bacteriol. 1970 Oct;104(1):13–18. doi: 10.1128/jb.104.1.13-18.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]