Abstract
Dendritic spines are of major importance in information processing and memory formation in central neurons. Estradiol has been shown to induce an increase of dendritic spine density on hippocampal neurons in vivo and in vitro. The neurotrophin brain-derived neurotrophic factor (BDNF) recently has been implicated in neuronal maturation, plasticity, and regulation of GABAergic interneurons. We now demonstrate that estradiol down-regulates BDNF in cultured hippocampal neurons to 40% of control values within 24 hr of exposure. This, in turn, decreases inhibition and increases excitatory tone in pyramidal neurons, leading to a 2-fold increase in dendritic spine density. Exogenous BDNF blocks the effects of estradiol on spine formation, and BDNF depletion with a selective antisense oligonucleotide mimics the effects of estradiol. Addition of BDNF antibodies also increases spine density, and diazepam, which facilitates GABAergic neurotransmission, blocks estradiol-induced spine formation. These observations demonstrate a functional link between estradiol, BDNF as a potent regulator of GABAergic interneurons, and activity-dependent formation of dendritic spines in hippocampal neurons.
Dendritic spines, the loci of excitatory synaptic transmission among central neurons, are associated with long-term functional plasticity in the central nervous system (1). It has been shown that estradiol causes a dramatic increase in dendritic spine density in cultured hippocampal pyramidal neurons (2), as it does in vivo (3). It was discovered recently that estradiol down-regulates glutamic acid decarboxylase (GAD) and its product, γ-aminobutyric acid (GABA), and subsequently, that miniature inhibitory postsynaptic currents (mIPSCs) are reduced in both size and frequency (4). This indicates that, by reducing the efficacy of inhibitory connections, estradiol causes an increase in the excitatory drive on pyramidal neurons, resulting in an activity-dependent rise of intracellular calcium and subsequent formation of new dendritic spines. The rise in spine density is mediated by phosphorylation of cAMP response element binding protein (5), which is most likely caused by an increase in free intracellular calcium concentration (6), resulting from an increase in network activity (7).
Brain derived neurotrophic factor (BDNF) is known to play an important role in neuronal development and plasticity in an activity-dependent manner (8). Although its effects are still not understood completely, it has been demonstrated that BDNF functions in the regulation of GABAergic phenotype during development of hippocampal neurons in culture (9, 10), as well as activity-dependent regulation of inhibition in cortical (11, 12) and hippocampal interneurons (13). The regulation of BDNF itself is related closely to activity (14), and mRNAs for both BDNF and GAD coexist in hippocampal interneurons (15). We therefore examined the hypothesis that BDNF is linked to the effects of estrogen and the control of GABAergic interneurons. We found that estradiol down-regulates BDNF expression in cultured hippocampal neurons, leading to a reduction in GAD and GABA and a subsequent increase in electrical activity and formation of new dendritic spines.
MATERIALS AND METHODS
Hippocampal Cultures.
Hippocampal neurons were prepared as described (2, 16). Brains were removed from 19- to 20-day-old Sprague–Dawley rat embryos and were placed in cold L15 medium containing 0.6% glucose and 15 μg/ml gentamicin. The hippocampus was dissected and was disaggregated mechanically by gentle trituration. Dissociated cells were plated onto 12-mm glass coverslips for immunostaining and electrophysiology (500,000 cells per well) or into 12-well plastic dishes for Western blotting (1.3 × 106 cells per well). These were coated with poly-l-lysine and were UV sterilized. The plating medium was Eagle’s MEM with 10% heat-inactivated horse serum, 5% fetal calf serum, 2 mM glutamine, 0.6% glucose, and 15 μg/ml gentamicin. Cells were incubated at 37°C with 8% CO2. The first change of medium (3–4 days after plating) included 50 μg/ml uridine and 20 μg/ml deoxyuridine to prevent glial cell overgrowth. The cultures then were fed 1–2 times per week with Eagle’s MEM containing 10% horse serum.
Dosing Regimen.
Two days before all experiments, cells were placed in serum-free media (N4 in DMEM with 0.1% ovalbumin). Cells were treated with water-soluble estradiol (0.01 μg/ml, Sigma), BDNF (5nM, Research Biochemicals), diazepam (10 μM), BDNF antibody (1 μg/ml, Santa Cruz Biotechnology), or oligonucleotides (5 μM). Antisense (AS) and reversed AS (RAS) phosphorothioate oligonucleotides (23-mers) were targeted to the BDNF translation initiation codon (−13 to +10). The oligonucleotide sequence for BDNF AS was 5′-GGATGGTCATCACTCTTCTCACC-3′ and for RAS was 5′-CCACTCTTCTCACTACTGGTAGG-3′. Oligonucleotides were supplied by Operon Technologies (Alameda, CA). Each treatment group consisted of at least two wells, and all experiments were repeated at least once with a different culture to verify that unique culture conditions did not contribute to the observed effects.
Immunofluorescence and Intensity Analysis.
Cells were fixed in 4% paraformaldehyde in PBS for 1 hr at room temperature, were washed, and were permeabilized with 0.1% saponin in blocking buffer (10% BSA/PBS) for 30 min. Coverslips were drained and inverted over microdrops of primary antibodies to GAD (Boehringer Mannheim), BDNF, NT-3 (Santa Cruz Biotechnology), or GABA (Sigma). Cells were incubated overnight at 4°C, then were washed in PBS and were incubated in secondary antibodies for 1hr at room temperature. Cells were mounted in Vectashield (Vector Laboratories) and were imaged on a confocal laser scanning microscope with a 100× 1.4 numerical aperture oil immersion lens. Identical confocal settings were used for each group. Random fields (15–20 fields) were selected from each group, and images were recorded for intensity analysis. The fluorescence intensity was calculated by using nih-image software. Comparisons were made by using t tests and ANOVAs for unpaired groups.
Western Blot Analysis.
Cell lysates were prepared by rinsing cultures grown in 12-well dishes with PBS and then were solubilized with the addition 1% SDS, 1 mM sodium vanadate, 0.5% Triton X-100, and 10 mM Tris⋅HCl (pH 7.4) and were boiled for 5 min. Samples (20 μg) were separated on a 14% SDS-polyacrylamide minigel (NOVEX, San Diego) before transferring to nitrocellulose. The membranes were incubated with rabbit polyclonal antisera to BDNF (Santa Cruz Biotechnology) in the presence of blocking solution (PBS/0.1% Tween 20/5% nonfat dry milk) overnight at 4°C. Incubations with a secondary antibody, donkey anti-rabbit IgG conjugated to horseradish peroxidase (Amersham), were performed for 1hr at room temperature. The signal was detected by enhanced chemiluminescence (ECL, Amersham) according to manufacturer’s instructions. Purified human BDNF (20–200 μg) was run on adjacent lanes to assay for BDNF concentration and antibody specificity. Human BDNF migrated more slowly (≈15 kDa) than rat BDNF (≈14 kDa), as has been reported (17).
Morphological Analysis of Dendritic Spines.
Cells were fixed in 4% paraformaldehyde for 1 hr at room temperature. Individual cells were stained by pressurized microinjection of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine dissolved in oil, as detailed elsewhere (16). Cells were imaged with a confocal laser scanning microscope as described above by using 2× zoom. Z sections (0.5 μm) were collected and were reconstructed serially by using LSM software (Zeiss). Single sections and reconstructions were used jointly to count the number of spines for measured lengths of dendrite in nih-image, ensuring that each spine was counted only once. No corrections for “hidden spines” were made. Spines were counted if they were <3–4 μm in length and possessed a mature “head.” Density was calculated for dendritic lengths of 50 μm. For each treatment, at least 12–15 cells were reconstructed, and 48–60 dendritic segments were measured.
Electrophysiology.
Cells were visualized in a phase-contrast, inverted microscope as described (6). In brief, cells were recorded with a CsCl-containing patch pipette in extracellular recording medium containing 130 mM NaCl, 4 mM KCl, 20 mM Hepes, 10 mM glucose, 3 mM CaCl2, 1 mM MgCl2, 1 μM tetrodotoxin, and 30 μM 6,7-dinitroquinoxaline-2,3-dione (to block excitatory synaptic currents). Under these conditions, miniature spontaneous postsynaptic currents are recorded. The GABAergic nature of these postsynaptic currents was verified by their disappearance in presence of the GABA-A receptor antagonist bicuculline (applied at 50 μM concentration in the recording chamber; data not shown). pH was adjusted to 7.4, and osmolarity was adjusted to 320 milliosmoles with sucrose. Signals were amplified with Axopatch 200 amplifier (Axon Instruments) and were stored and analyzed with pclamp-6 Axograph (Axon Instruments) and origin (Microcal Software, Northhampton, MA) software packages. Comparisons were made by using unpaired t tests or ANOVA tests, as applicable.
RESULTS
Estradiol Down-Regulates BDNF.
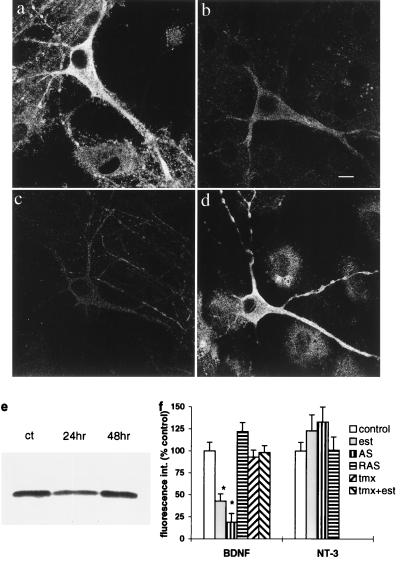
We first examined the effects of estradiol on BDNF immunoreactivity in 2–3 week old hippocampal cultures. At this time in culture, GABAergic connections are fully mature and function as inhibitory synapses (10). Estradiol-treated cultures displayed a time-dependent decrease in BDNF fluorescence, which peaked at 24 hr and recovered within 48 hr of exposure (Fig. 1 a and b). This transient decrease in BDNF immunoreactivity was confirmed with Western blot analysis, showing a decrease in the BDNF band to 64% of control within 24 hr of exposure to estradiol and returning to normal levels at 48 hr (Fig. 1e). Blots were stripped and stained for MAP2, which showed no change (not shown). Quantitative immunofluorescence analysis indicated that estradiol lowered BDNF immunoreactivity to 68% of control at 12 hr (control, 136.5 ± 6.0 arbitrary intensity units; estradiol, 92 ± 14.3), 40% at 24 hr (control, 158 ± 5.7; estradiol, 63.6 ± 7.5; t test, P < 0.01), 55% at 36 hr (control, 122.6 ± 5.2; estradiol, 68 ± 6.7; P < 0.01) and returned to 100% of control values within 48 hr of exposure. The effects of estradiol on BDNF were mediated by an estrogen receptor, as they were blocked by the estrogen receptor antagonist tamoxifen, which had no effect of its own on BDNF immunofluorescence (Fig. 1f). Estradiol was selective for BDNF in that it had no significant effect on neurotrophin-3 (NT-3) levels in the cultured neurons after 24 hr of exposure (Fig. 1f).
Figure 1.
BDNF immunoreactivity is down-regulated by estradiol in cultured hippocampal neurons. (a) Control. (b) Estradiol, 24 hr. AS-treated (c) cells exhibit a marked reduction in staining for BDNF whereas RAS-treated cells (d) display normal staining. (Bar = 10 μm.) (e) Typical western blot of control cultures (left) and those collected after 24- and 48-hr exposure to estradiol. Lanes were loaded with equal protein, and a single BDNF band appeared near 14 kDa. On average (3 experiments), the density of the BDNF band at 24 hr was 64% of control. (f) BDNF fluorescence staining intensity measurements from several experiments were averaged and presented as a percent of control, which was run separately for each experiment. Bars represent means ± SEM, and asterisks represent statistically significant difference from control at P < 0.01, Student’s t test. The bars, from left to right, are control, estradiol (est), BDNF-AS, BDNF-RAS, tamoxifen (tmx), and tamoxifen + estradiol (tmx + est). Only the estradiol and the BDNF-AS caused a significant reduction in BDNF staining. On the right are measurements of neurotrophin-3 (NT-3) fluorescence intensity, showing little effect of estradiol or the BDNF-AS.
BDNF Up-Regulates GAD and GABA.
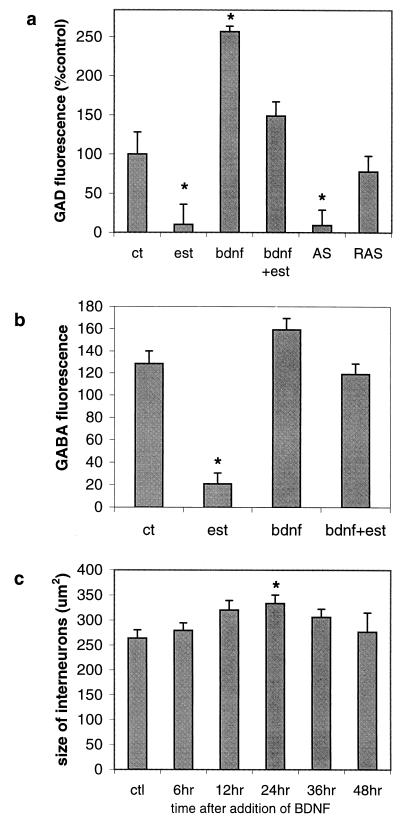
Recently, we proposed that estradiol induces spine formation by down-regulating GABA synthesis, thereby increasing the overall excitatory tone in the network of cultured hippocampal neurons (4). This is similar to the effects seen when inhibition is blocked (16). We therefore examined a possible connection between estradiol and BDNF in regulating GAD expression. As shown previously, estradiol produced a 90% reduction in GAD-positive particles, an effect that peaked at 24 hrs of exposure (Fig. 2 a and b). Exogenously applied BDNF increased the density of GAD and GABA immunoreactive particles, (Fig. 2 c and d and Fig. 3 a and b) and caused an increase in the size of GABAergic cell somata that was statistically significant at 24 hr (Fig. 3c). Estradiol alone did not change the cell size significantly (224.5 ± 22 μm2). Of most importance, exogenously applied BDNF blocked the effects of estradiol on GAD and GABA immunoreactivity (Fig. 2d and Fig. 3 a and b), indicating that down-regulation of BDNF mediates the effects of estradiol on GABAergic neurons.
Figure 2.
BDNF and estradiol have opposing effects on GAD immunoreactivity. (a and b) The number of GAD-positive fluorescent particles decreased significantly after 24 hr of estradiol treatment [control, 55.9 ± 15.5 units (a); estradiol, 5.6 ± 1.5 units (b)]. (c) GAD immunoreactive levels increased significantly with BDNF treatment (227.3 ± 17.6). (d) Cultures exposed to BDNF and estradiol simultaneously did not exhibit decreased GAD staining seen with estradiol alone (83.6 ± 19). (Bar = 10 μm.) Similar results were obtained with GABA staining (images not shown).
Figure 3.
BDNF mimics effects of estradiol on GAD and GABA immunoreactivity. (a) GAD fluorescence intensity is expressed as a percent of control. Estradiol significantly reduces, and BDNF enhances, GAD immunofluorescence. BDNF-AS mimics the estradiol-induced decrease in GAD whereas RAS is ineffective. (b) Estradiol causes a marked reduction in GABA immunofluorescence, as expected from its action on GAD. BDNF increases GABA, although not significantly, but blocks the estradiol-induced decrease in GABA. (c) A significant increase in the size of GAD positive interneurons was observed at 24 hr of exposure to BDNF. The other time points did not yield significant effects. Estradiol did not decrease the cell size significantly (224.5 ± 22 μm; data not shown).
AS Experiments.
If BDNF blocks the effect of estradiol on GABAergic cells, depletion of BDNF may be expected to have effects similar to those of estradiol. We attempted to reduce endogenous levels of the peptide by using a selective AS oligonucleotide for BDNF. An RAS routinely was used as a negative control. The effectiveness of AS treatment was evident in that a marked reduction in BDNF immunoreactivity was seen in AS-treated cultures that was not seen in the RAS group (Fig. 1 c and d). The AS treatment was specific for BDNF in that it caused a nonsignificant increase in NT-3 staining (Fig. 1f). We then stained the cultures for GAD and GABA and found that BDNF-AS caused a large reduction in immunoreactivity for both antigens, compared with control and RAS-treated cultures (Fig. 3 a and b).
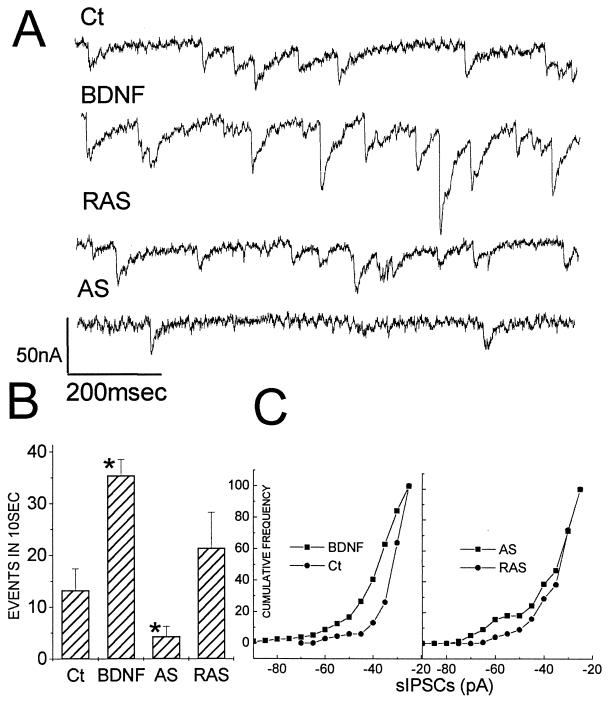
A functional patch clamp analysis was conducted to determine the physiological consequences of modulating BDNF levels. Cells exposed to 5 nM BDNF for 24 hr expressed a marked increase in the frequency and size of spontaneous miniature IPSCs (recorded in tetrodotoxin and 6,7-dinitroquinoxaline-2,3-dione, to block miniature excitatory postsynaptic currents). Cells exposed to BDNF-AS under the same conditions for 24 hr displayed a significant reduction in the frequency of mIPSCs (Fig. 4) without affecting their magnitudes. The AS treatment did not affect miniature excitatory postsynaptic currents (recorded in presence of bicuculline and tetrodotoxin and the absence of 6,7-dinitroquinoxaline-2,3-dione; data not shown). The RAS-treated cells were not different from untreated controls in any of the parameters tested. Also, there were no differences in the duration of the mIPSCs among the four groups. Because the largest effect of AS treatment was on the frequency of mIPSCs rather than their amplitude, it is likely that BDNF-AS affects presynaptic release properties of mIPSCs. Thus, BDNF-AS behaved much like estradiol (4) in reducing GABAergic inhibitory tone in the cultures.
Figure 4.
BDNF regulates spontaneous mIPSCs in cultured hippocampal neurons. (a) mIPSCs recorded for 1 sec in control, BDNF treated, RAS, and AS oligonucleotide-treated cultures. Note the difference in size and frequency of mIPSCs in the different conditions. (b) Total number of events in 10-sec recording intervals for all of the cells recorded in the study (n = 6 for control, n = 8 for BDNF, n = 6 for RAS, and n = 14 for AS). The BDNF group had significantly higher rates of spontaneous mIPSC discharges, and the AS group had significantly lower rates of discharges (ANOVA followed by individual t tests; ∗, P < 0.01). (c) Cumulative histograms of the mIPSCs sizes in the four groups: left, control and BDNF; right, RAS and AS. BDNF produced significantly larger mIPSCs than the corresponding control group (t = 4.54, P < 0.01) whereas there was no difference in size of mIPSCs between the AS and RAS groups.
Analysis of Dendritic Spines.
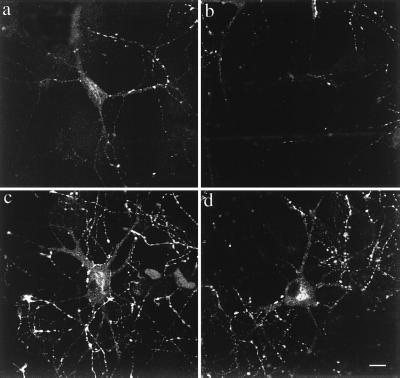
Estradiol is believed to cause formation of dendritic spines by reducing inhibition in the cultured neuronal network (4). We replicated previous experiments (2) to show that estradiol caused a large increase in dendritic spine density (mean of seven experiments; Fig. 5 a and b; Table 1). Strikingly, exogenously applied BDNF totally suppressed the effect of estradiol on dendritic spine density and had a small effect of its own to reduce dendritic spine density to 84% of control (Table 1). These results were mimicked by diazepam, which facilitates GABAergic neurotransmission. Furthermore, exposure of cultures to BDNF-AS mimicked the effects of estradiol on dendritic spine density whereas BDNF-RAS was ineffective (Fig. 5 c and d; Table 1). The effects of estradiol and BDNF-AS were not additive, indicating that they may share a common mechanism of action. Exogenous BDNF reversed the effect of BDNF-AS on spine formation, indicating that AS blocked formation of BDNF but did not block the receptor and downstream mechanisms for regulation of spine density (Table 1). Consistent with this, an antibody to BDNF significantly increased spine density to 142% of control when applied to the cultures (Table 1). This is similar to the effects observed with BDNF-AS and is likely caused by neutralization of secreted BDNF.
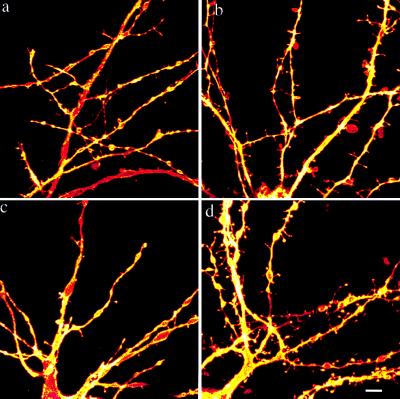
Figure 5.
BDNF-AS mimics the estradiol response. Cultures were dosed in serum free media for 2 days. (a) Control. (b) Estradiol. (c) RAS. (d) BDNF-AS. Images are reconstructions of z-series from single 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-labeled neurons, taken on the confocal laser-scanning microscope. (Bar = 10 μm.)
Table 1.
BDNF and estradiol interact to produce changes in spine density
| Experiment | Control | Estradiol | Treatment groups | ||
|---|---|---|---|---|---|
| 1 | 7.79 ± 0.32 | 13.78 ± 0.43* | BDNF, 6.33 ± 0.23 | BDNF + estradiol, 5.88 ± 0.26 | |
| 2 | 8.46 ± 0.43 | 13.1 ± 0.5* | BDNF, 7.23 ± 0.47 | BDNF + k252a, 8.71 ± 0.42 | |
| 3 | 8.47 ± 0.38 | 14.31 ± 0.59* | BDNF, 7.19 ± 0.38 | BDNF + k252a, 11.19 ± 0.52* | K252a, 12.7 ± 0.57* |
| 8.47 ± 0.38 | 14.31 ± 0.59* | BDNF, 7.19 ± 0.38 | BDNF AS, 13.4 ± 0.46* | BDNF RAS, 8.49 ± 0.35 | |
| 4 | 9.1 ± 0.29 | 13.68 ± 0.4* | BDNF, 7.48 ± 0.4 | BDNF AS, 14.14 ± 0.45* | BDNF RAS, 8.68 ± 0.49 |
| 5 | 7.62 ± 0.42 | 11.41 ± 0.8* | Diazepam + estradiol, 7.39 ± 0.34 | Diazepam, 6.51 ± 0.4 | |
| 6 | 8.59 ± 0.39 | 13.16 ± 0.06* | BDNF-antibody, 12.18 ± 0.63* |
Summary of six experiments measuring <250 dendritic segments from ≈50 cells in each group. Values are spines per 50-μm dendritic segment. Asterisks denote significance from control at P < 0.01 by using unpaired Student’s t test. In experiment 1, note that BDNF by itself causes a 19% reduction in dendritic spine density but totally blocks the effect of estradiol. Experiments 2 and 3 illustrate that both the trk-B receptor antagonist, K252a, and BDNF-AS mimic the effects of estradiol. BDNF by itself does not block the effect of the antagonist, unlike its effect on estradiol, indicating that K252a acts downstream of BDNF. In experiment 5, diazepam blocks the effects of estradiol on spine density, indicating that the GABAergic link is important in regulating spine formation. Finally, in experiment 6, BDNF-Ab mimics the effects of estradiol.
It has been established that trkB is the specific receptor subtype for BDNF (18) and is blocked by the antagonist K252a. If the effects of BDNF are mediated by the activation of trkB receptor, then blockade of trkB should mimic the effects of BDNF-AS and BDNF-antibody on dendritic spine formation. Moreover, it should block the reduction in spine density produced by BDNF. Indeed, incubation of the cultures with K252a caused a rise of dendritic spine density to 149% of control (from 8.5 ± 0.38 in control to 12.7 ± 0.57 spines per 50-μm dendrite), similar to the 153% rise produced by estradiol and 158% caused by BDNF-AS in the same experiment. When incubated together with K252a, BDNF reversed its effect from a reduction in spine density (7.19 ± 0.38 spines per 50-μm dendrite) to a slight increase (11.19 ± 0.52), indicating that BDNF acts via trkB.
DISCUSSION
It has been shown that estradiol induces formation of dendritic spines in hippocampal cultured neurons by lowering GABA levels and decreasing inhibitory activity (4). The present experiments demonstrate that these effects are mediated by a transient down-regulation of BDNF, a potent regulator of GABAergic interneurons. BDNF by itself caused an increase in GAD and GABA levels and enhanced inhibitory synaptic tone as measured by an increase in the frequency of mIPSCs. In contrast, when BDNF was depleted by a specific antisense oligonucleotide, mIPSC frequency was reduced markedly. These findings confirm previous studies suggesting BDNF affects the development, morphology, and function of inhibitory interneurons (11). Additionally, we observed that BDNF AS treatment, as well as antibodies to BDNF, promote spine growth. Thus, we propose that the lowering of BDNF after estradiol treatment is responsible for the reduction in GABAergic transmission, which ultimately leads to formation of new dendritic spines.
Modulation of BDNF levels by estradiol may operate at the transcriptional level because of the sequence homology existing between the nuclear estrogen response element and regions within exon V of the BDNF gene (19). Estrogen-bound receptor may bind to these regions and may directly regulate BDNF expression levels. However, we observed that BDNF levels decreased in all neurons in the culture whereas only inhibitory interneurons are thought to contain the estrogen receptor (α). Thus, BDNF would be down-regulated directly by estrogen-bound receptor only in these cells. One explanation for this involves recent evidence suggesting that BDNF is transported anterogradely in axons and is released from terminals (20) and that BDNF may regulate its own production by binding to trkB in a positive feedback manner (21). As BDNF is lowered by estradiol in inhibitory neurons, lack of binding at trkB receptors on other cells then may serve to further down-regulate their BDNF synthesis as well. Another possibility is that the newly discovered estrogen receptor (β) is present in all hippocampal neurons and mediates the broad effects of estradiol; the distribution of this receptor has yet to be described fully.
The effects of estradiol on BDNF, GABAergic activity, and spine formation are transient, which is consistent with the physiology of hormonal cycling. After a brief period of enhanced activity and formation of new spines, BDNF returns to normal levels, restoring inhibitory tone. This is not surprising, as BDNF was shown to be elevated under conditions of excess activity (22). Thus, a feedback mechanism may exist whereby increases in action potential discharge of pyramidal neurons lead to BDNF and thus GABA production, reducing excitability of the network. The means by which this may occur are unknown. However, it was observed that phosphorylation of cAMP response element binding protein (CREB) is necessary for estradiol-induced increases in spine density (5). This most likely is induced by an increase in intracellular calcium concentration (6) and network activity (7). Because BDNF is elevated under conditions of excess activity (22), and BDNF transcription is regulated by a cAMP response element binding protein dependent mechanism (23), it is thus tempting to hypothesize that the recovery of BDNF levels after 48 hr is mediated by CREB, completing the feedback loop.
The occurrence of a BDNF feedback loop controlling spine growth in hippocampal neurons could serve two purposes. First, if the new spines are not needed as permanent connections, they may be resorbed as the excitatory drive is balanced. Second, if any of the new spines develop into permanent connections, BDNF now would be available to help regulate presynaptic protein levels (24) and may facilitate transmission at these newly formed synapses (25), thus ensuring their maturation. BDNF has been implicated in regulation of neural development and plasticity, notably long term potentiation (26). Indeed, it has been shown that suppression of BDNF with antisense oligonucleotides causes impairment of long term potentiation (27). Thus, the recovery of BDNF 2 days after estradiol treatment in culture may explain, in part, the facilitation of long term potentiation seen late in the afternoon on the day of proestrous when synapse density in the hippocampus is highest (28). We cannot rule out that estradiol and BDNF may have additional effects not examined here. However, it is clear that there is a strong link between estradiol and BDNF in regulation of cultured GABAergic neurons that leads to activity-dependent formation of new dendritic spines.
Acknowledgments
The authors thank Dr. Bai Lu of the National Institute of Child Health and Human Development for critical comments on the manuscript, Drs. T.S. Reese and S.B. Andrews for their support, and the Light Imaging Facility, National Institute of Neurological Disorders and Stroke, for use of the confocal microscope. This work was supported in part by a U.S.–Israel Binational Science Foundation grant to M.S.
ABBREVIATIONS
- BDNF
brain-derived neurotrophic factor
- GAD
glutamic acid decarboxylase
- GABA
γ-aminobutyric acid
- mIPSC
miniature inhibitory postsynaptic current
- AS
antisense
- RAS
reversed AS
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Harris K, Kater S. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 2.Murphy D D, Segal M. J Neurosci. 1996;16:4059–4068. doi: 10.1523/JNEUROSCI.16-13-04059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woolley C, McEwen B S. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy D D, Cole N B, Greenberger V, Segal M. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy D D, Segal M. Proc Natl Acad Sci USA. 1997;94:1482–1487. doi: 10.1073/pnas.94.4.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore A N, Waxham M N, Dash P K. J Biol Chem. 1996;271:14214–14220. doi: 10.1074/jbc.271.24.14214. [DOI] [PubMed] [Google Scholar]
- 7.Diesseroth K, Bito H, Tsien R. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- 8.Lu B, Figarov A. Rev Neurosci. 1997;8:1–12. doi: 10.1515/revneuro.1997.8.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Berninger B, Marty S, Zafra F, Berzaghi M, Thoenen H, Lindholm D. Development. 1995;121:2327–2335. doi: 10.1242/dev.121.8.2327. [DOI] [PubMed] [Google Scholar]
- 10.Marty S, Berninger B, Carroll P, Thoenen H. Neuron. 1996;16:565–570. doi: 10.1016/s0896-6273(00)80075-6. [DOI] [PubMed] [Google Scholar]
- 11.Marty S, Berzaghi M, Berninger B. Trends Neurosci. 1997;20:198–202. doi: 10.1016/s0166-2236(96)01026-0. [DOI] [PubMed] [Google Scholar]
- 12.Rutherford L C, DeWan A, Lauer H M, Turrigiano G G. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartrup J T, Moorman J M, Newberry N R. NeuroReport. 1997;8:3791–3794. doi: 10.1097/00001756-199712010-00027. [DOI] [PubMed] [Google Scholar]
- 14.Zafra F, Castren E, Thoenen H, Lindholm D. Proc Natl Acad Sci USA. 1991;88:10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zachrisson O, Falkenberg T, Lindefors N. Mol Brain Res. 1995;36:169–173. doi: 10.1016/0169-328x(95)00281-v. [DOI] [PubMed] [Google Scholar]
- 16.Papa M, Segal M. Neuroscience. 1995;71:1005–1011. doi: 10.1016/0306-4522(95)00490-4. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi N, Bedard A, Hincke M, Tetzlaff W. Eur J Neurosci. 1996;8:1018–1029. doi: 10.1111/j.1460-9568.1996.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 18.Barbacid M. Ann NY Acad Sci. 1995;766:442–458. doi: 10.1111/j.1749-6632.1995.tb26693.x. [DOI] [PubMed] [Google Scholar]
- 19.Sohrabji F, Miranda R, Torand-Allorand C D. Proc Natl Acad Sci USA. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altar C A, Cai N, Bliven T, Juhasz M, Conner J M, Acheson A L, Lindsay R M, Wiegand S J. Nature (London) 1997;389:856–859. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 21.Canossa M, Griesbeck O, Berninger B, Campana G, Kolbeck R, Thoenen H. Proc Natl Acad Sci USA. 1997;94:13279–13286. doi: 10.1073/pnas.94.24.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kornblum H I, Sankar R, Shin D H, Wasterlain C G, Gall C M. Mol Brain Res. 1997;44:219–228. doi: 10.1016/s0169-328x(96)00224-0. [DOI] [PubMed] [Google Scholar]
- 23.Tao X, Finkbeiner S, Arnold D B, Shaywitz A J, Greenberg M E. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 24.Takei N, Sasaoka K, Inoue K, Takahashi M, Endo Y, Hatanaka H. J Neurochem. 1997;68:370–375. doi: 10.1046/j.1471-4159.1997.68010370.x. [DOI] [PubMed] [Google Scholar]
- 25.Stoop R, Poo M. Prog Brain Res. 1996;109:359–364. doi: 10.1016/s0079-6123(08)62118-4. [DOI] [PubMed] [Google Scholar]
- 26.Figurov A, Pozzo-Miller L D, Olafsson P, Wang T, Lu B. Nature (London) 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 27.Ma Y L, Wang H L, Wu H C, Wei C L, Lee E. Neuroscience. 1998;82:957–967. doi: 10.1016/s0306-4522(97)00325-4. [DOI] [PubMed] [Google Scholar]
- 28.Warren S G, Humphreys A G, Juraska J M, Greenough W T. Brain Res. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]