Abstract
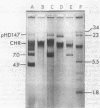
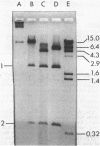
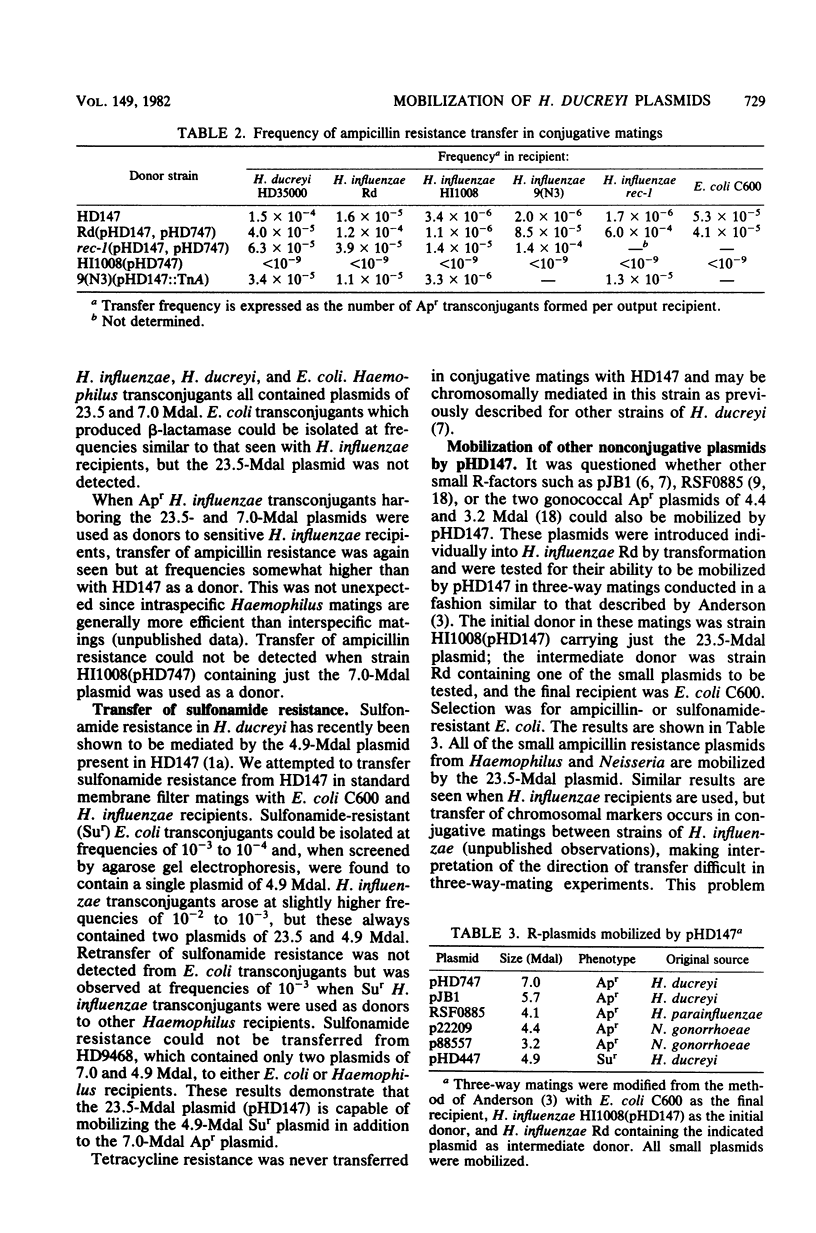
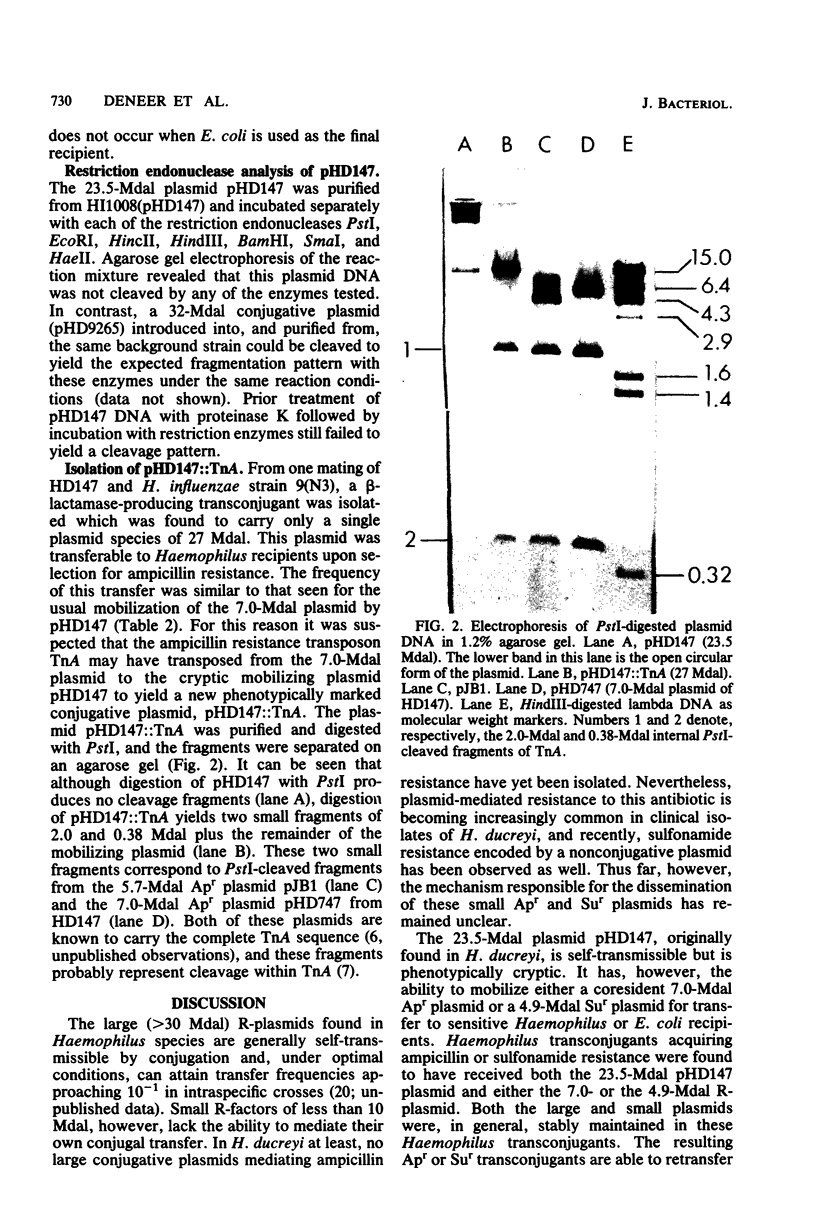
A clinical isolate of Haemophilus ducreyi was found to harbor three plasmids: a 23.5-megadalton (Mdal) phenotypically cryptic plasmid, a 7.0-Mdal ampicillin resistance plasmid, and a 4.0-Mdal sulfonamide resistance plasmid. The two smaller plasmids were transferable by conjugation to Haemophilus recipients, but only if the donor cell harbored the 23.5-Mdal plasmid as well, indicating that this large plasmid had mobilizing capabilities. Transfer was also possible to Escherichia coli recipients. Haemophilus influenzae transconjugants which had acquired both the 23.5-Mdal plasmid and one of the R-plasmids could subsequently retransfer the R-plasmid to other Haemophilus recipients at higher frequencies. A derivative of the 23.5 Mdal plasmid was isolated which was shown by restriction endonuclease analysis to contain an ampicillin resistance transposon and to have retained its conjugative ability.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER H. E., LEIDY G. Determination of inherited traits of H. influenzae by desoxyribonucleic acid fractions isolated from type-specific cells. J Exp Med. 1951 Apr 1;93(4):345–359. doi: 10.1084/jem.93.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albritton W. L., Bendler J. W., Setlow J. K. Plasmid transformation in Haemophilus influenzae. J Bacteriol. 1981 Feb;145(2):1099–1101. doi: 10.1128/jb.145.2.1099-1101.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albritton W. L., Brunton J. L., Slaney L., MacLean I. Plasmid-mediated sulfonamide resistance in Haemophilus ducreyi. Antimicrob Agents Chemother. 1982 Jan;21(1):159–165. doi: 10.1128/aac.21.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron E. S., Saz A. K., Kopecko D. J., Wohlhieter J. A. Transfer of plasmid-borne beta-lactamase in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1977 Aug;12(2):270–280. doi: 10.1128/aac.12.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G. D., Blackman E. Y., Sparling P. F. High-frequency conjugal transfer of a gonococcal penicillinase plasmid. J Bacteriol. 1980 Sep;143(3):1318–1324. doi: 10.1128/jb.143.3.1318-1324.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton J. L., Maclean I., Ronald A. R., Albritton W. L. Plasmid-mediated ampicillin resistance in Haemophilus ducreyi. Antimicrob Agents Chemother. 1979 Feb;15(2):294–299. doi: 10.1128/aac.15.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., De Graaff J., Seibert D., Falkow S. Plasmid-linked ampicillin resistance in haempohilus influenza type b. Infect Immun. 1975 Aug;12(2):404–410. doi: 10.1128/iai.12.2.404-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbane J. J., Malamy M. H. F factor mobilization of non-conjugative chimeric plasmids in Escherichia coli: general mechanisms and a role for site-specific recA-independent recombination at orV1. J Mol Biol. 1980 Oct 15;143(1):73–93. doi: 10.1016/0022-2836(80)90125-4. [DOI] [PubMed] [Google Scholar]
- Laufs R., Kaulfers P. M., Jahn G., Teschner U. Molecular characterization of a small Haemophilus influenzae plasmid specifying beta-lactamase and its relationship to R factors from Neisseria gonorrhoeae. J Gen Microbiol. 1979 Mar;111(1):223–231. doi: 10.1099/00221287-111-1-223. [DOI] [PubMed] [Google Scholar]
- MacLean I. W., Bowden G. H., Albritton W. L. TEM-type beta-lactamase production in Haemophilus ducreyi. Antimicrob Agents Chemother. 1980 May;17(5):897–900. doi: 10.1128/aac.17.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K., Murray N. E. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease III of Haemophilus influenzae and restriction endonuclease I of Escherichia coli. J Mol Biol. 1975 Nov 5;98(3):551–564. doi: 10.1016/s0022-2836(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., Joshi V. R., Allison D. P. Molecular basis for the transformation defects in mutants of Haemophilus influenzae. J Bacteriol. 1972 Jun;110(3):1171–1180. doi: 10.1128/jb.110.3.1171-1180.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarowicz A., Siwińska M. Inhibition of transformation and transfection in Haemophilus influenzae Rd9 by lysogeny. J Bacteriol. 1977 Jan;129(1):22–29. doi: 10.1128/jb.129.1.22-29.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Elwell L. P., Falkow S. Molecular characterization of two beta-lactamase-specifying plasmids isolated from Neisseria gonorrhoeae. J Bacteriol. 1977 Aug;131(2):557–563. doi: 10.1128/jb.131.2.557-563.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Falkow S. Conjugal transfer of R plasmids in Neisseria gonorrhoeae. Nature. 1977 Apr 14;266(5603):630–631. doi: 10.1038/266630a0. [DOI] [PubMed] [Google Scholar]
- Saunders J. R., Elwell L. P., Falkow S., Sykes R. B., Richmond M. H. beta-lactamases and R-plasmids of Haemophilus influenzae. Scand J Infect Dis Suppl. 1978;(13):16–22. [PubMed] [Google Scholar]
- Saunders J. R., Sykes R. B. Transfer of a plasmid-specified beta-lactamase gene from Haemophilus influenzae. Antimicrob Agents Chemother. 1977 Feb;11(2):339–344. doi: 10.1128/aac.11.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Beattie K. L., Kimball R. F. A complex of recombination and repair genes in Haemophilus influenzae. J Mol Biol. 1972 Jul 21;68(2):361–378. doi: 10.1016/0022-2836(72)90218-5. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Transfer factors in Escherichia coli with particular regard to their incidence in enteropathogenic strains. J Gen Microbiol. 1970 Aug;62(3):287–299. doi: 10.1099/00221287-62-3-287. [DOI] [PubMed] [Google Scholar]
- Stuy J. H. Plasmid transfer in Haemophilus influenzae. J Bacteriol. 1979 Aug;139(2):520–529. doi: 10.1128/jb.139.2.520-529.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Mayer L. W., Young F. E. Physical map of the conjugal plasmid of Neisseria gonorrhoeae. Infect Immun. 1980 Jul;29(1):181–185. doi: 10.1128/iai.29.1.181-185.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]