Abstract
Hydrolytic activities of isolated membrane fractions of Escherichia coli against chromogenic substrates, p-nitrophenyl ester and beta-naphthyl ester derivatives of N-substituted amino acids, were investigated by spectrophotometric and electrophoretic methods. Although detergents were absolutely necessary for the solubilization of enzymes, the amount of solubilized activities was increased by adding salt, such as NaCl or KCl. Two esterases were identified and separated by PAGE and by chromatography of the solubilized proteins in the presence of detergent. One hydrolyzed the alanine derivatives preferentially, whereas the other was mainly active on phenylalanine derivatives. Only the first was inactivated by diisopropyl fluorophosphate, a serine hydrolase inhibitor. Whereas the chymotrypsin-like enzyme was equally distributed between the inner and the outer membrane, the alanine activity was only detected in the inner membrane. They were both resistant to extraction with high salt concentrations, indicating their integral association with membranes. A study of the accessibility of these enzymes to their substrate in membrane vesicles with known polarity suggests that both alanine and phenylalanine activities are localized near the external surface of the cytoplasmic (inner) membrane. However, the phenylalanine activity (chymotrypsin-like enzyme) appears to be deeply buried inside the outer membrane. Because of its insensitivity to diisopropyl fluorophosphate, this last esterase seems to be distinct from the previously isolated periplasmic endopeptidase, protease I, which is also a chymotrypsin-like enzyme.
Full text
PDF





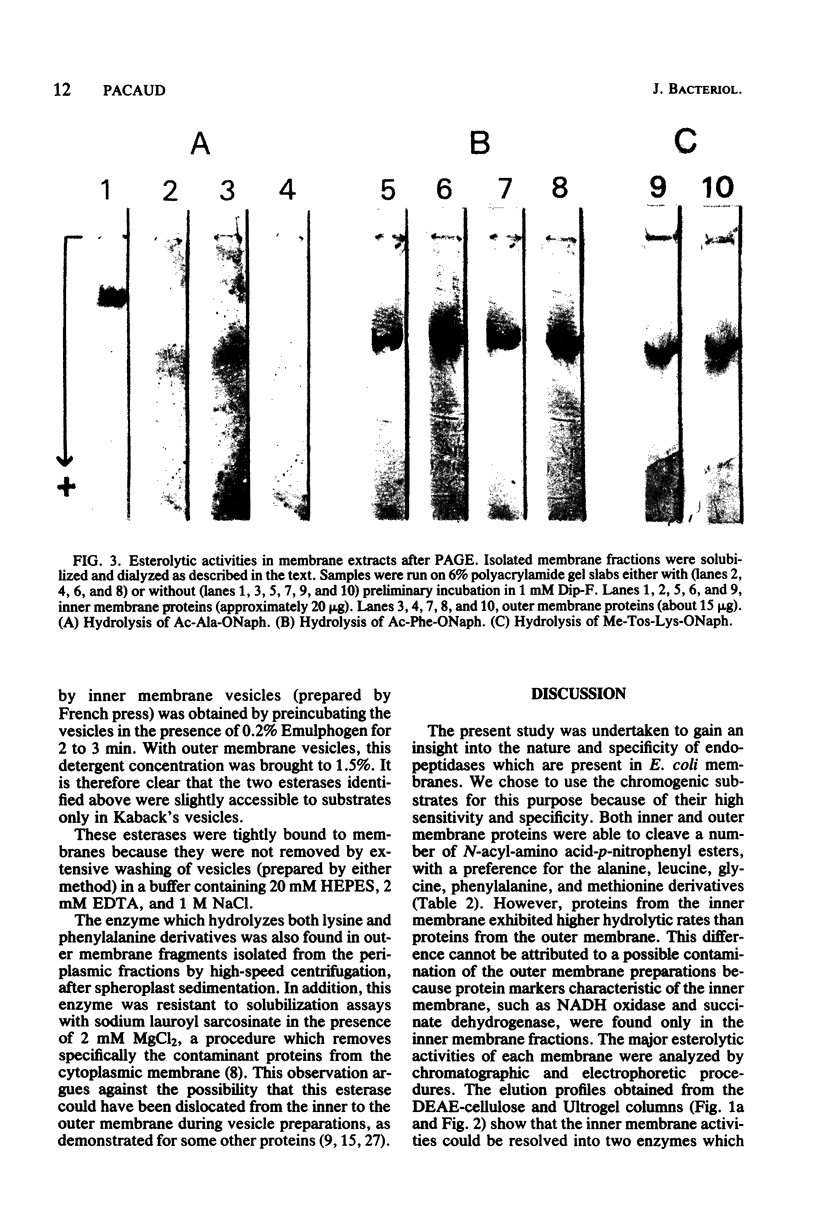


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K. H., Staehelin L. A. Orientation of membrane vesicles from Escherichia coli as detected by freeze-cleave electron microscopy. J Bacteriol. 1974 Feb;117(2):888–899. doi: 10.1128/jb.117.2.888-899.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Adsorption of bacteriophages to adhesions between wall and membrane of Escherichia coli. J Virol. 1968 Apr;2(4):346–356. doi: 10.1128/jvi.2.4.346-356.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Zipser D. Purification and characterization of protease III from Escherichia coli. J Biol Chem. 1979 Jun 10;254(11):4698–4706. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davis B. D., Tai P. C. The mechanism of protein secretion across membranes. Nature. 1980 Jan 31;283(5746):433–438. doi: 10.1038/283433a0. [DOI] [PubMed] [Google Scholar]
- De Leij L., Witholt B. Structural heterogeneity of the cytoplasmic and outer membranes of Escherichia coli. Biochim Biophys Acta. 1977 Nov 15;471(1):92–104. doi: 10.1016/0005-2736(77)90396-0. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M. Orientation of membrane vesicles from Escherichia coli prepared by different procedures. J Membr Biol. 1974;15(1):15–28. doi: 10.1007/BF01870079. [DOI] [PubMed] [Google Scholar]
- Hare J. F., Olden K., Kennedy E. P. Heterogeneity of membrane vesicles from Escherichia coli and their subfractionation with antibody to ATPase. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4843–4846. doi: 10.1073/pnas.71.12.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Sato T., Yura T. Synthesis and assembly of the membrane proteins in E. coli. Cell. 1977 Jul;11(3):551–559. doi: 10.1016/0092-8674(77)90073-3. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Antigenic architecture of membrane vesicles from Escherichia coli. Biochemistry. 1979 Apr 17;18(8):1422–1426. doi: 10.1021/bi00575a005. [DOI] [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Molecular structure of membrane vesicles from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3148–3152. doi: 10.1073/pnas.75.7.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud M. Purification of protease II from Escherichia coli by affinity chromatography and separation of two enzyme species from cells harvested at late log phase. Eur J Biochem. 1976 Apr 15;64(1):199–204. doi: 10.1111/j.1432-1033.1976.tb10288.x. [DOI] [PubMed] [Google Scholar]
- Pacaud M., Richaud C. Protease II from Escherichia coli. Purification and characterization. J Biol Chem. 1975 Oct 10;250(19):7771–7779. [PubMed] [Google Scholar]
- Pacaud M., Sibilli S., Bras G. Protease I from Escherichia coli. Some physicochemical properties and substrate specificity. Eur J Biochem. 1976 Oct 1;69(1):141–151. doi: 10.1111/j.1432-1033.1976.tb10867.x. [DOI] [PubMed] [Google Scholar]
- Pacaud M., Uriel J. Isolation and some propeties of a proteolytic enzyme from Escherichia coli (protease I). Eur J Biochem. 1971 Dec 10;23(3):435–442. doi: 10.1111/j.1432-1033.1971.tb01638.x. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Smyth C. J., Siegel J., Salton M. R., Owen P. Immunochemical analysis of inner and outer membranes of Escherichia coli by crossed immunoelectrophoresis. J Bacteriol. 1978 Jan;133(1):306–319. doi: 10.1128/jb.133.1.306-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C., Nozaki Y., Reynolds J. A., Makino S. Molecular characterization of proteins in detergent solutions. Biochemistry. 1974 May 21;13(11):2369–2376. doi: 10.1021/bi00708a021. [DOI] [PubMed] [Google Scholar]
- Watson D. H., Sherratt D. J. In vivo proteolytic cleavage of colicins requires specific receptor binding. Nature. 1979 Mar 22;278(5702):362–364. doi: 10.1038/278362a0. [DOI] [PubMed] [Google Scholar]
- Yamato I., Futai M., Anraku Y., Nonomura Y. Cytoplasmic membrane vesicles of Escherichia coli. II. Orientation of the vesicles studied by localization of enzymes. J Biochem. 1978 Jan;83(1):117–128. doi: 10.1093/oxfordjournals.jbchem.a131882. [DOI] [PubMed] [Google Scholar]



