Abstract
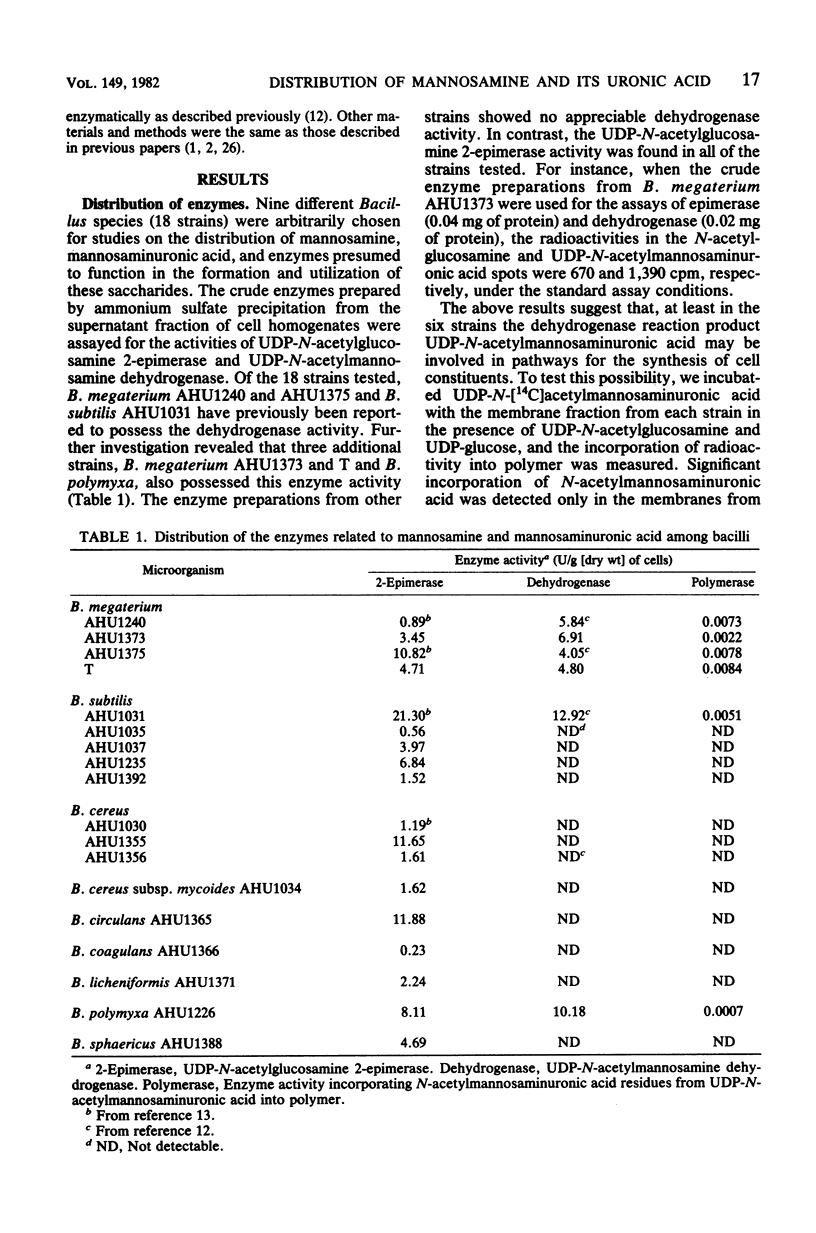
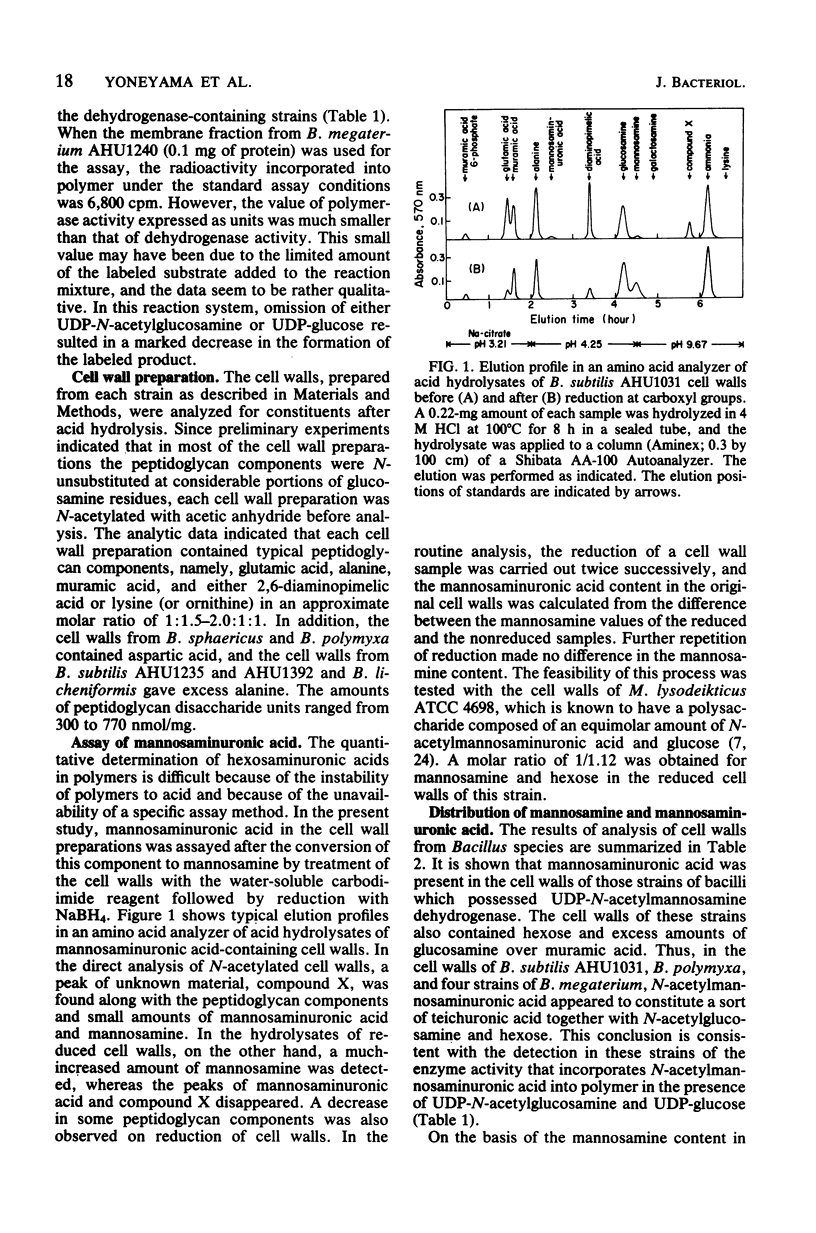
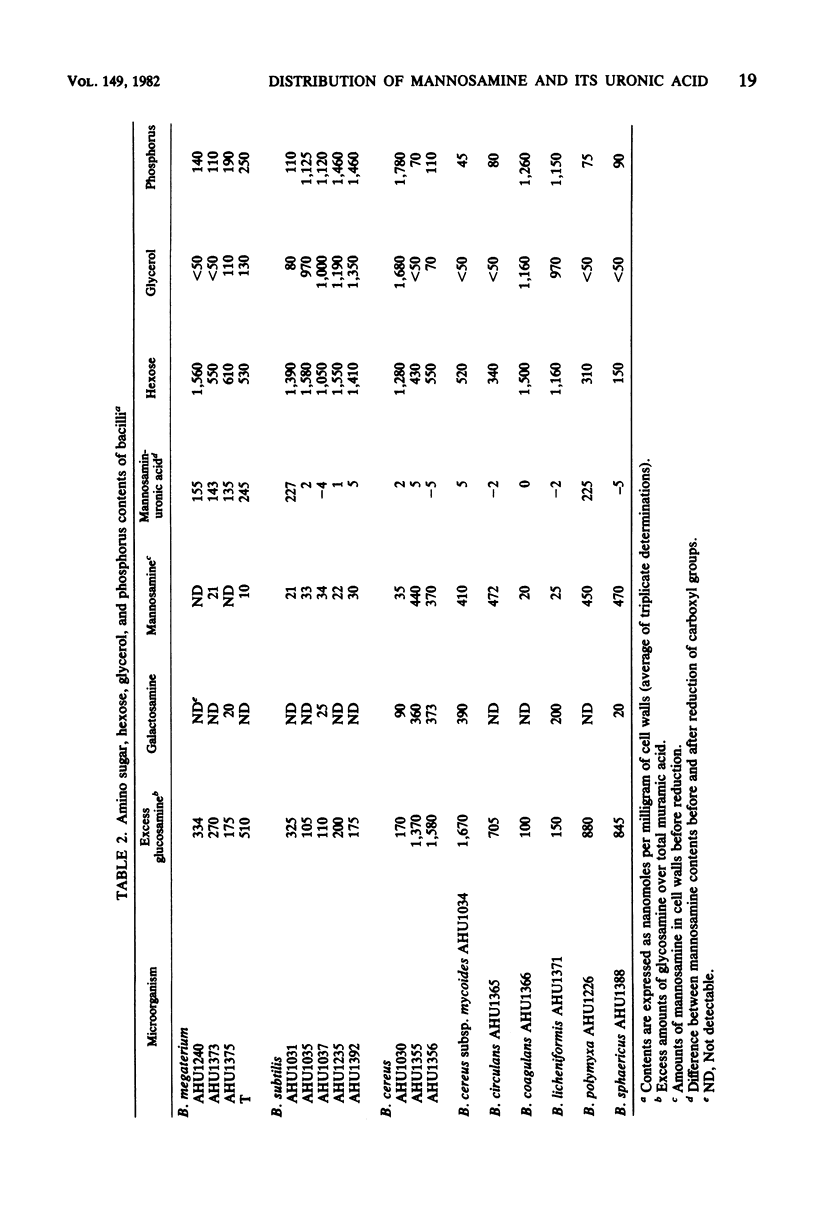
The distribution of mannosamine, mannosaminuronic acid, and the enzymes responsible for the formation of these saccharides was studied in nine species (18 strains) of Bacillus. Whereas UDP-N-acetylglucosamine 2-epimerase activity was detected in all of the strains examined, UDP-N-acetylmannosamine dehydrogenase, as well as the activity incorporating N-acetylmannosaminuronic acid residues from UDP-N-acetylmannosaminuronic acid into polymer, was found only in four strains of B. megaterium and one strain each of B. subtilis and B. polymyxa. The cell walls prepared from the six above-named strains were shown to contain mannosaminuronic acid in amounts of 135 to 245 nmol/mg. In contrast, mannosamine had a wide distribution. The cell walls from two strains of B. cereus and one strain each of B. circulans, B. polymyxa, B. sphaericus, and B. cereus subsp. mycoides contained mannosamine in amounts of 370 to 470 nmol/mg. In addition, the cell walls from five strains of B. subtilis, two strains of B. megaterium, and one strain each of B. cereus. B. coagulans, and B. licheniformis also contained this amino sugar in amounts as small as 10 to 35 nmol/mg. On the basis of analytical data, it is suggested that the mannosamine present in small amounts may be a common constituent of linkage units between peptidoglycan and other cell wall components such as glycerol teichoic acid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. J., BADDILEY J., BUCHANAN J. G. Further studies on the teichoic acid from Bacillus subtilis walls. Biochem J. 1961 Aug;80:254–261. doi: 10.1042/bj0800254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano K., Hazama S., Araki Y., Ito E. Isolation and characterization of structural components of Bacillus cereus AHU 1356 cell walls. Eur J Biochem. 1977 May 16;75(2):513–522. doi: 10.1111/j.1432-1033.1977.tb11552.x. [DOI] [PubMed] [Google Scholar]
- Araki Y., Nakatani T., Nakayama K., Ito E. Occurrence of N-nonsubstituted glucosamine residues in peptidoglycan of lysozyme-resistant cell walls from Bacillus cereus. J Biol Chem. 1972 Oct 10;247(19):6312–6322. [PubMed] [Google Scholar]
- Coley J., Archibald A. R., Baddiley J. A linkage unit joining peptidoglycan to teichoic acid in Staphylococcus aureus H. FEBS Lett. 1976 Jan 15;61(2):240–242. doi: 10.1016/0014-5793(76)81047-2. [DOI] [PubMed] [Google Scholar]
- Coley J., Tarelli E., Archibald A. R., Baddiley J. The linkage between teichoic acid and peptidoglycan in bacterial cell walls. FEBS Lett. 1978 Apr 1;88(1):1–9. doi: 10.1016/0014-5793(78)80594-8. [DOI] [PubMed] [Google Scholar]
- Hase S., Matsushima Y. Structural studies on a glucose-containing polysaccharide obtained from cell walls of Micrococcus lysodeikticus. 3. Determination of the structure. J Biochem. 1972 Nov;72(5):1117–1128. doi: 10.1093/oxfordjournals.jbchem.a129999. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Araki Y., Ito E. Occurrence of glucosamine residues with free amino groups in cell wall peptidoglycan from bacilli as a factor responsible for resistance to lysozyme. J Bacteriol. 1973 Feb;113(2):592–598. doi: 10.1128/jb.113.2.592-598.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heptinstall J., Coley J., Ward P. J., Archibald A. R., Baddiley J. The linkage of sugar phosphate polymer to peptidoglycan in walls of Micrococcus sp. 2102. Biochem J. 1978 Feb 1;169(2):329–336. doi: 10.1042/bj1690329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungerer K. D., Tipper D. J. Cell wall polymers of Bacillus sphaericus 9602. I. Structure of the vegetative cell wall peptidoglycan. Biochemistry. 1969 Sep;8(9):3577–3587. doi: 10.1021/bi00837a013. [DOI] [PubMed] [Google Scholar]
- Kawamura T., Ishimoto N., Ito E. Enzymatic synthesis of uridine diphosphate N-acetyl-D-mannosaminuronic acid. J Biol Chem. 1979 Sep 10;254(17):8457–8465. [PubMed] [Google Scholar]
- Kawamura T., Kimura M., Yamamori S., Ito E. Enzymatic formation of uridine diphosphate N-acetyl-D-mannosamine. J Biol Chem. 1978 May 25;253(10):3595–3601. [PubMed] [Google Scholar]
- Kondo S., Iguchi T., Hisatsune K. Lipopolysaccharides of group F, a new group of vibrios. Biochem Biophys Res Commun. 1980 Nov 28;97(2):437–442. doi: 10.1016/0006-291x(80)90283-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Lee C. J., Fraser B. A. The structures of the cross-reactive types 19 (19F) and 57 (19A) pneumococcal capsular polysaccharides. J Biol Chem. 1980 Jul 25;255(14):6847–6853. [PubMed] [Google Scholar]
- Liau D. F., Melly M. A., Hash J. H. Surface polysaccharide from Staphylococcus aureus M that contains taurine, D-aminogalacturonic acid, and D-fucosamine. J Bacteriol. 1974 Sep;119(3):913–922. doi: 10.1128/jb.119.3.913-922.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T. Y., Gotschlich E. C., Jonssen E. K., Wysocki J. R. Studies on the meningococcal polysaccharides. I. Composition and chemical properties of the group A polysaccharide. J Biol Chem. 1971 May 10;246(9):2849–2858. [PubMed] [Google Scholar]
- Lugowski C., Romanowska E. Enterobacterial common antigen: isolation from Shigella sonnei, purification and immunochemical characterization. Eur J Biochem. 1978 Nov 2;91(1):89–97. doi: 10.1111/j.1432-1033.1978.tb20941.x. [DOI] [PubMed] [Google Scholar]
- Mayer H. D-Mannosaminuronsäure-Baustein des K7-antigens von Escherichia coli. Eur J Biochem. 1969 Mar;8(1):139–145. doi: 10.1111/j.1432-1033.1969.tb00506.x. [DOI] [PubMed] [Google Scholar]
- Murazumi N., Sasaki Y., Okada J., Araki Y., Ito E. Biosynthesis of glycerol teichoic acid in Bacillus cereus: formation of linkage unit disaccharide on a lipid intermediate. Biochem Biophys Res Commun. 1981 Mar 31;99(2):504–510. doi: 10.1016/0006-291x(81)91773-3. [DOI] [PubMed] [Google Scholar]
- Männel D., Mayer H. Isolation and chemical characterization of the enterobacterial common antigen. Eur J Biochem. 1978 May 16;86(2):361–370. doi: 10.1111/j.1432-1033.1978.tb12318.x. [DOI] [PubMed] [Google Scholar]
- PERKINS H. R. A polymer containing glucose and aminohexuronic acid isolated from the cell walls of micrococcus lysodeikticus. Biochem J. 1963 Mar;86:475–483. doi: 10.1042/bj0860475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr T. E., Levy G. N., Stark N. J., Anderson J. S. Initial reactions in biosynthesis of teichuronic acid of Micrococcus lysodeikticus cell walls. J Biol Chem. 1977 May 25;252(10):3460–3465. [PubMed] [Google Scholar]
- Sasaki Y., Araki Y., Ito E. Structure of linkage region between glycerol teichoic acid and peptidoglycan in Bacillus cereus AHU 1030 cell walls. Biochem Biophys Res Commun. 1980 Sep 30;96(2):529–534. doi: 10.1016/0006-291x(80)91388-1. [DOI] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- Torii M., Sakakibara K., Kuroda K. Occurrence of 2-amino-2-deoxy-hexuronic acids as constituents of Vibrio parahaemolyticus K15 antigen. Eur J Biochem. 1973 Sep 3;37(3):401–405. doi: 10.1111/j.1432-1033.1973.tb02999.x. [DOI] [PubMed] [Google Scholar]
- Vann W. F., Liu T. Y., Robbins J. B. Bacillus pumilus polysaccharide cross-reactive with meningococcal group A polysaccharide. Infect Immun. 1976 Jun;13(6):1654–1662. doi: 10.1128/iai.13.6.1654-1662.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. C., Park J. T. Chemical characterization of a new surface antigenic polysaccharide from a mutant of Staphylococcus aureus. J Bacteriol. 1971 Nov;108(2):874–884. doi: 10.1128/jb.108.2.874-884.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadomae T., Ohno N., Miyazaki T. On the phosphate linkages and the structure of a disaccharide unit of the type-specific polysaccharide of Pneumococcus type XIX. Carbohydr Res. 1979 Oct;75:191–198. doi: 10.1016/s0008-6215(00)84638-8. [DOI] [PubMed] [Google Scholar]



