Abstract
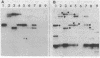
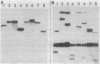
We have physically and genetically characterized 20 symbiotic and 20 auxotrophic mutants of Rhizobium meliloti, the nitrogen-fixing symbiont of alfalfa (Medicago sativa), isolated by transposon Tn5 mutagenesis. A "suicide plasmid" mutagenesis procedure was used to generate TN-5-induced mutants, and both auxotrophic and symbiotic mutants were found at a frequency of 0.3% among strains containing random TN5 insertions. Two classes of symbiotic mutants were isolated: 4 of the 20 formed no nodules at all (Nod-), and 16 formed nodules which failed to fix nitrogen (Fix-). We used a combination of physical and genetic criteria to determine that in most cases the auxotrophic and symbiotic phenotypes could be correlated with the insertion of a single Tn5 elements. Once the Tn5 element was inserted into the R. meliloti genome, the frequency of its transposition to a new site was approximately 10-8 and the frequency of precise excision was less than 10-9. In approximately 25% of the mutant strains, phage Mu DNA sequences, which originated from the suicide plasmid used to generate the Tn5 transpositions, were also found in the R. meliloti genome contiguous with Tn5. These later strains exhibited anomalous conjugation properties, and therefore we could not correlate the symbiotic phenotype with a Tn5 insertion. In general, we found that both physical and genetic tests were required to fully characterize transposon-induced mutations.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Knight E., Jr ATP-dependent reduction of azide and HCN by N2-fixing enzymes of Azotobacter vinelandii and Clostridium pasteurianum. Biochim Biophys Acta. 1967 May 16;139(1):69–90. doi: 10.1016/0005-2744(67)90114-3. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade H. M., Signer E. R. Genetic mapping of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1977 May;74(5):2076–2078. doi: 10.1073/pnas.74.5.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G. E., Ausubel F. M., Cannon F. C. Physical map of chromosomal nitrogen fixation (nif) genes of Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2866–2870. doi: 10.1073/pnas.76.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Shaw K. J., Berg C. M. Escherichia coli K-12 auxotrophs induced by insertion of the transposable element Tn5. Genetics. 1979 Jul;92(3):741–747. doi: 10.1093/genetics/92.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet F., Silva B., van Montagu M., Schell J. Transfer of RP4::mu plasmids to Agrobacterium tumefaciens. Plasmid. 1978 Sep;1(4):446–455. doi: 10.1016/0147-619x(78)90003-3. [DOI] [PubMed] [Google Scholar]