Abstract
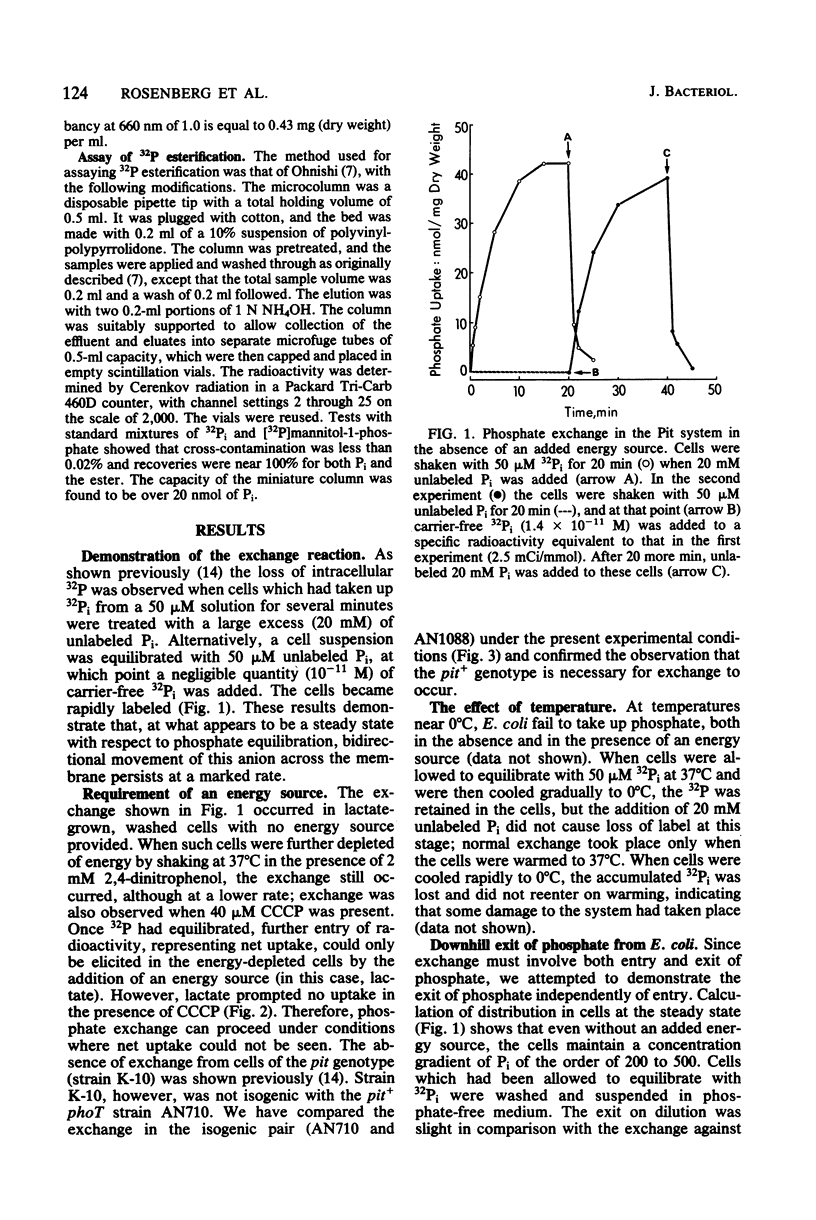
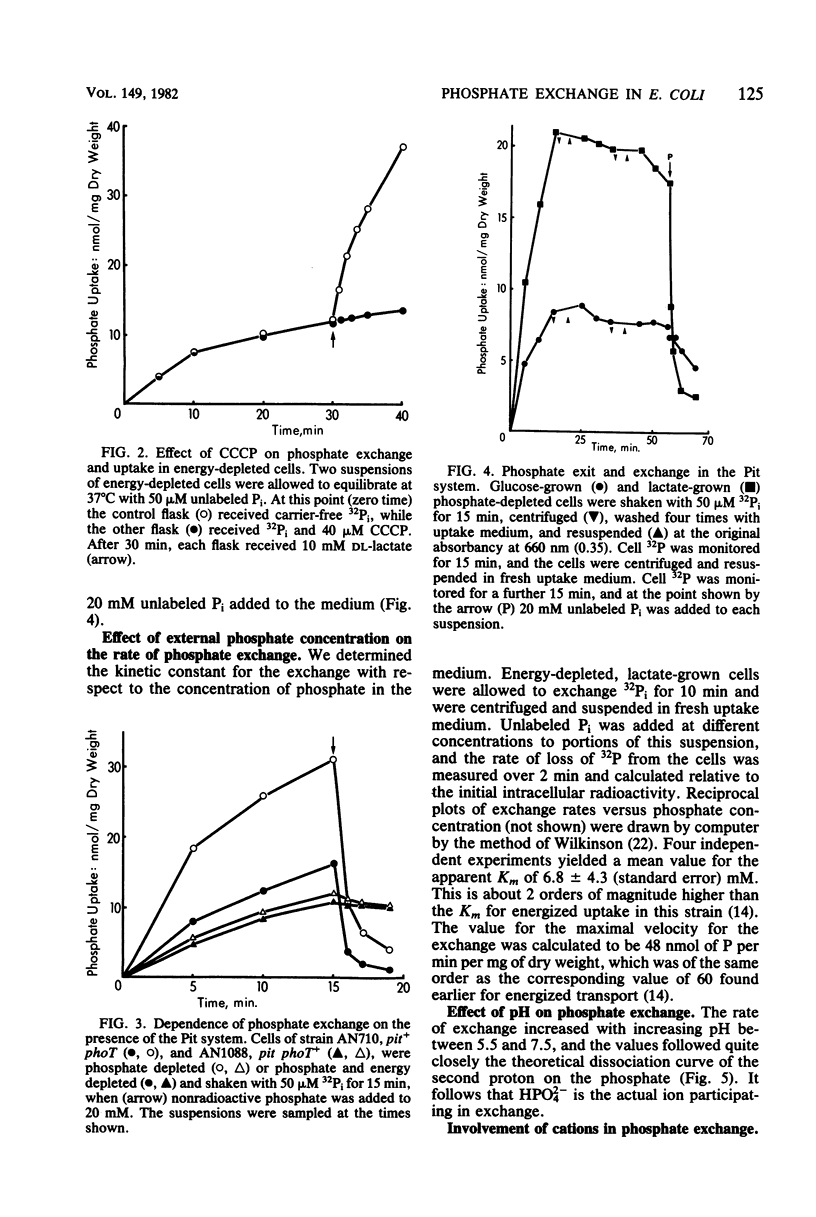
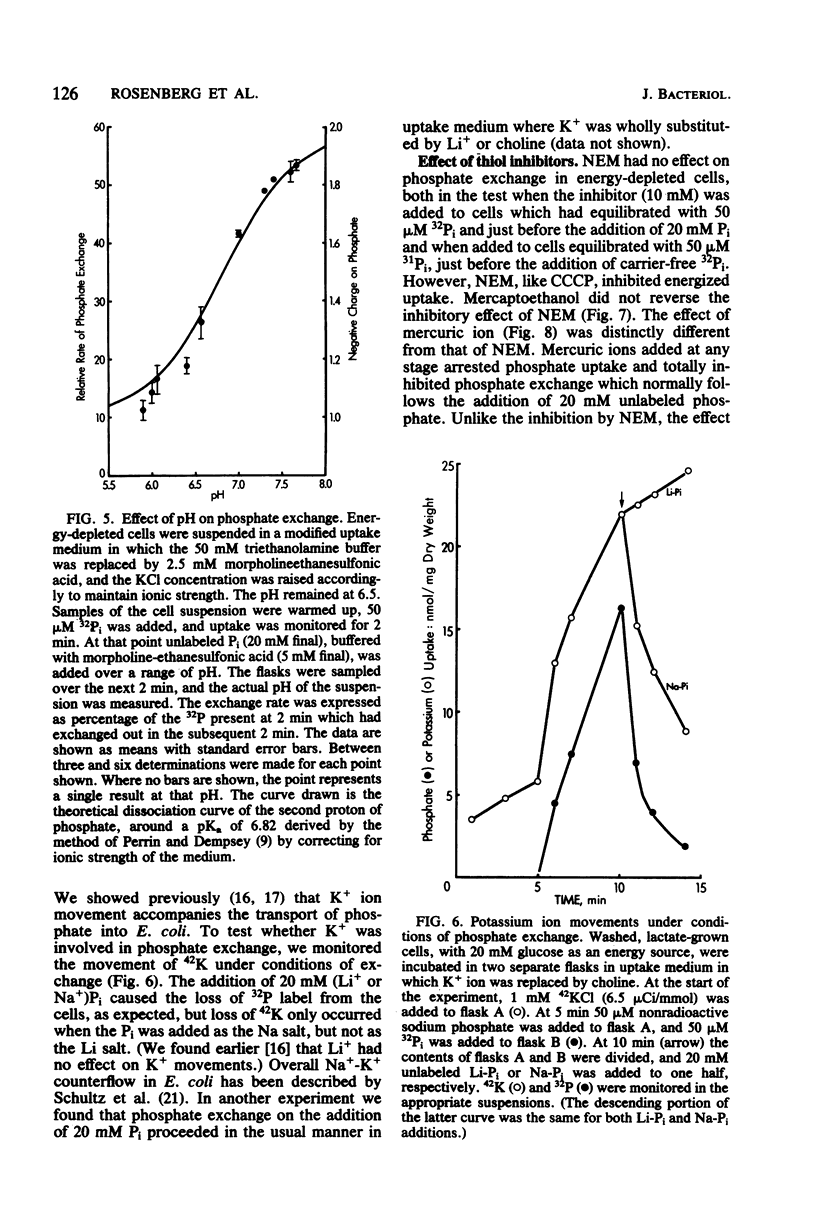
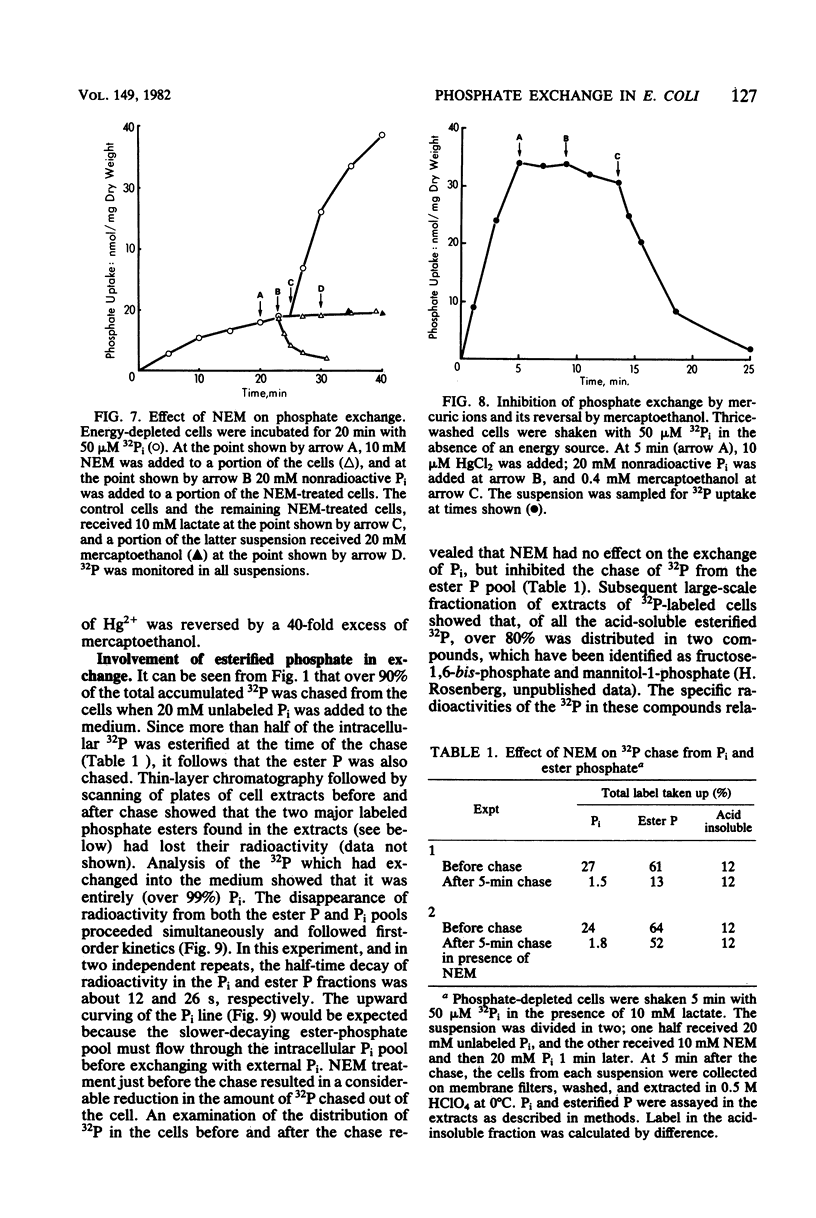
The Pit system of phosphate transport in Escherichia coli catalyzes a rapid exchange between the external inorganic phosphate and internal phosphate pools, including some ester phosphates which are in rapid equilibrium with the internal Pi pool. Unlike net energized uptake, the Pi exchange proceeds in energy-depleted cells in the presence of uncouplers and is not accompanied by the movement of potassium ions. In the absence of externally added phosphate, the exit of Pi from the cells is insignificant. The apparent Km for external Pi in the exchange reaction is about 7 mM (2 orders of magnitude higher than that of energized uptake), but the maximal velocity is about the same. The exchange is temperature sensitive and is affected by thiol reagents. The combined observations suggest the operation of a facilitator which is part of the Pit system. The exchange is repressed in cells grown on glucose and other phosphotransferase system substrates, but not in cells grown on other carbohydrate sources. The repression can be reversed by the addition of cyclic AMP to the medium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cockburn M., Earnshaw P., Eddy A. A. The stoicheiometry of the absorption of protons with phosphate and L-glutamate by yeasts of the genus Saccharomyces. Biochem J. 1975 Mar;146(3):705–712. doi: 10.1042/bj1460705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feucht B. U., Saier M. H., Jr Fine control of adenylate cyclase by the phosphoenolpyruvate:sugar phosphotransferase systems in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1980 Feb;141(2):603–610. doi: 10.1128/jb.141.2.603-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. A proposed common allosteric mechanism for active transport, muscle contraction, and ribosomal translocation. Proc Natl Acad Sci U S A. 1969 Sep;64(1):267–274. doi: 10.1073/pnas.64.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Hinkle P. C. Studies of the beta-galactoside transporter in inverted membrane vesicles of Escherichia coli. I. Symmetrical facilitated diffusion and proton gradient-coupled transport. J Biol Chem. 1977 Nov 10;252(21):7657–7661. [PubMed] [Google Scholar]
- MITCHELL P. Transport of phosphate across the osmotic barrier of Micrococcus pyogenes; specificity and kinetics. J Gen Microbiol. 1954 Aug;11(1):73–82. doi: 10.1099/00221287-11-1-73. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Performance and conservation of osmotic work by proton-coupled solute porter systems. J Bioenerg. 1973 Jan;4(1):63–91. doi: 10.1007/BF01516051. [DOI] [PubMed] [Google Scholar]
- Ohnishi S. T. A new method of separating inorganic orthophosphate from phosphoric esters and anhydrides by an immobilized catalyst column. Anal Biochem. 1978 May;86(1):201–213. doi: 10.1016/0003-2697(78)90335-4. [DOI] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Escherichia coli adenylate cyclase complex: regulation by the proton electrochemical gradient. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1099–1103. doi: 10.1073/pnas.76.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. The Escherichia coli adenylate cyclase complex: activation by phosphoenolpyruvate. J Supramol Struct. 1978;9(2):219–230. doi: 10.1002/jss.400090207. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Cox G. B., Butlin J. D., Gutowski S. J. Metabolite transport in mutants of Escherichia coli K12 defective in electron transport and coupled phosphorylation. Biochem J. 1975 Feb;146(2):417–423. doi: 10.1042/bj1460417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H., Gerdes R. G., Chegwidden K. Two systems for the uptake of phosphate in Escherichia coli. J Bacteriol. 1977 Aug;131(2):505–511. doi: 10.1128/jb.131.2.505-511.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H., Gerdes R. G., Harold F. M. Energy coupling to the transport of inorganic phosphate in Escherichia coli K12. Biochem J. 1979 Jan 15;178(1):133–137. doi: 10.1042/bj1780133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H. Transport of iron into bacterial cells. Methods Enzymol. 1979;56:388–394. doi: 10.1016/0076-6879(79)56036-4. [DOI] [PubMed] [Google Scholar]
- Russell L. M., Rosenberg H. Linked transport of phosphate, potassium ions and protons in Escherichia coli. Biochem J. 1979 Oct 15;184(1):13–21. doi: 10.1042/bj1840013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. M., Rosenberg H. The nature of the link between potassium transport and phosphate transport in Escherichia coli. Biochem J. 1980 Jun 15;188(3):715–723. doi: 10.1042/bj1880715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ S. G., EPSTEIN W., SOLOMON A. K. CATION TRANSPORT IN ESCHERICHIA COLI. IV. KINETICS OF NET K UPTAKE. J Gen Physiol. 1963 Nov;47:329–346. doi: 10.1085/jgp.47.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr Bacterial phosphoenolpyruvate: sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977 Dec;41(4):856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter D., Mindlin A. J. Dual influx model of thiogalactoside accumulation in Escherichia coli. J Biol Chem. 1969 Apr 10;244(7):1808–1816. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R., Bennett R. L., Malamy M. H. Inorganic phosphate transport in Escherichia coli: involvement of two genes which play a role in alkaline phosphatase regulation. J Bacteriol. 1973 Feb;113(2):529–539. doi: 10.1128/jb.113.2.529-539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R., Malamy M. H. Characterization of two genetically separable inorganic phosphate transport systems in Escherichia coli. J Bacteriol. 1980 Oct;144(1):356–365. doi: 10.1128/jb.144.1.356-365.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J. T., Pincock A., Bronskill P. M. Site of energy coupling in the carrier mechanism for beta-galactoside transport. Biochim Biophys Acta. 1971 Mar 9;233(1):176–188. doi: 10.1016/0005-2736(71)90370-1. [DOI] [PubMed] [Google Scholar]


