Abstract
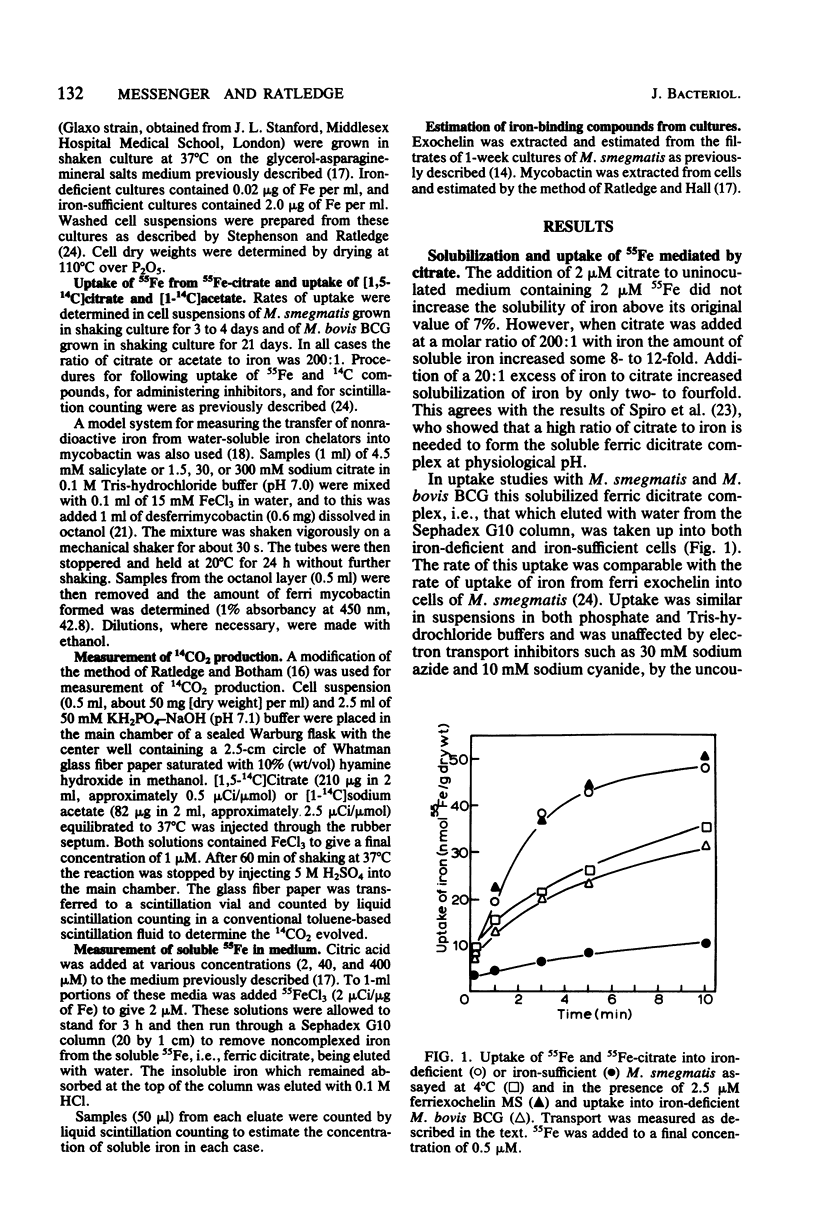
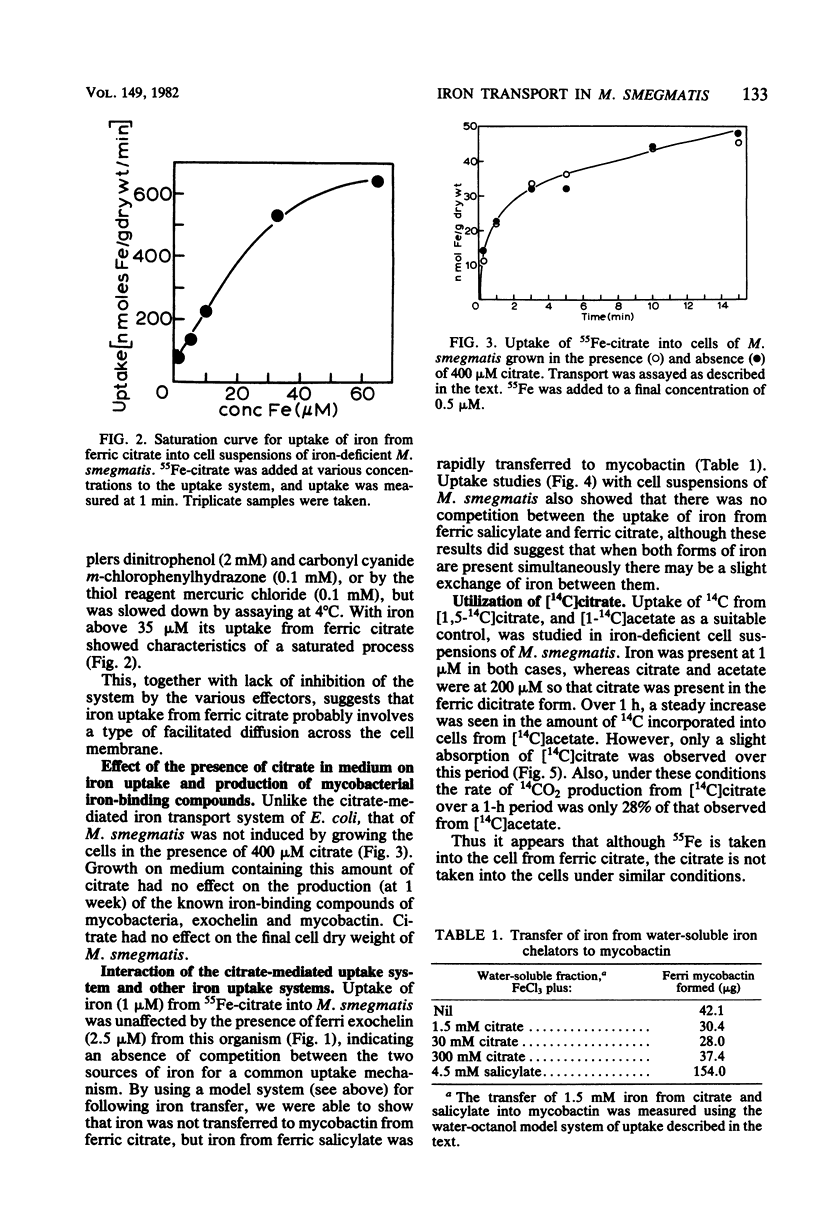
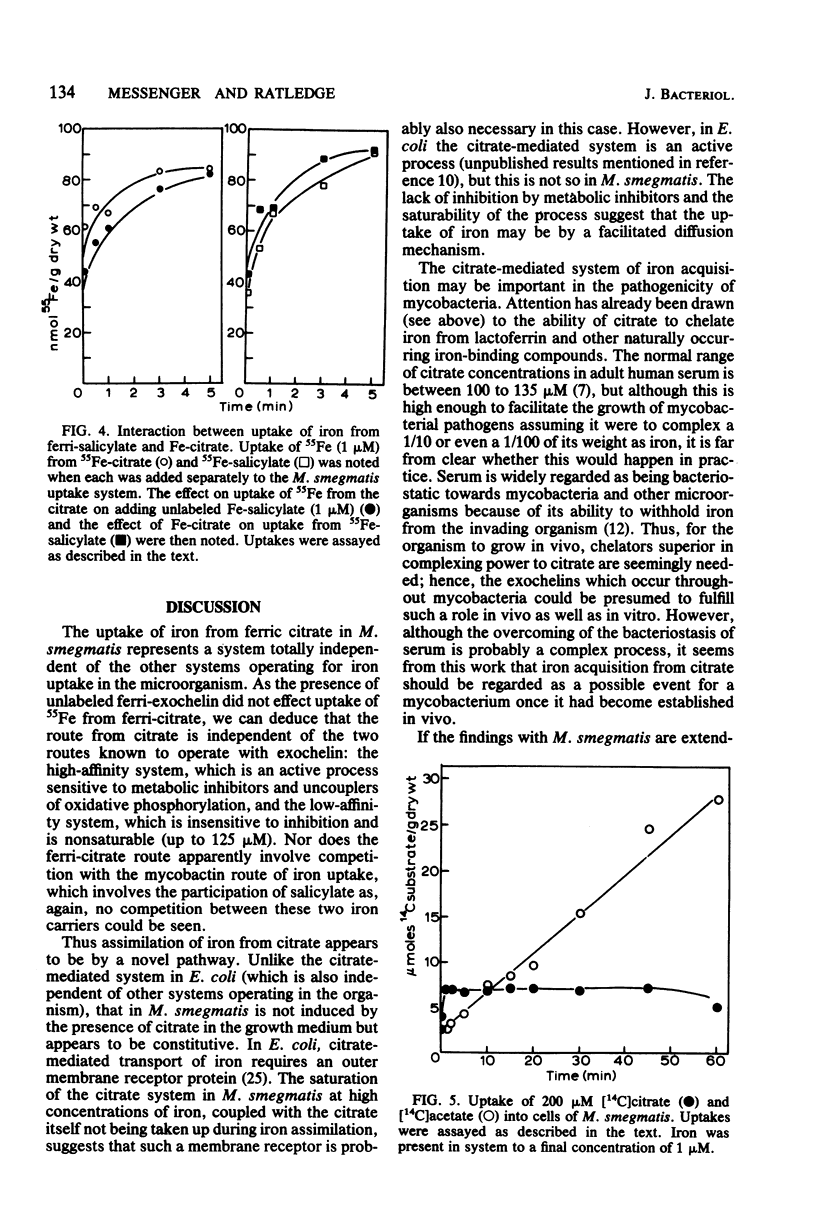
In mycobacterial growth medium 40 to 400 microM citrate was required to solubilize 2 microM 55Fe. This solubilized 55Fe was taken up into both iron-deficient and iron sufficient washed cell suspensions of Mycobacterium smegmatis and Mycobacterium bovis BCG. Although the 55Fe was taken up into the cell, the citrate was not. The uptake system with M. smegmatis was not inhibited by electron transport inhibitors, uncouplers of oxidative phosphorylation, or thiol reagents and was saturable with iron at approximately 35 microM. The system was independent of the iron transport systems already known to exist in M. smegmatis: i.e., the two exochelin routes of assimilation as well as the mycobactin-salicylate system. It was not induced by the presence of 400 microM citrate in the growth medium, nor did the presence of citrate in the medium affect the production of either exochelin or mycobactin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Leibman A., Zweier J. Stoichiometric and site characteristics of the binding of iron to human transferrin. J Biol Chem. 1978 Mar 25;253(6):1930–1937. [PubMed] [Google Scholar]
- Archibald F. S., DeVoe I. W. Iron acquisition by Neisseria meningitidis in vitro. Infect Immun. 1980 Feb;27(2):322–334. doi: 10.1128/iai.27.2.322-334.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates G. W., Billups C., Saltman P. The kinetics and mechanism of iron (3) exchange between chelates and transferrin. I. The complexes of citrate and nitrilotriacetic acid. J Biol Chem. 1967 Jun 25;242(12):2810–2815. [PubMed] [Google Scholar]
- Bishop J. G., Schanbacher F. L., Ferguson L. C., Smith K. L. In vitro growth inhibition of mastitis-causing coliform bacteria by bovine apo-lactoferrin and reversal of inhibition by citrate and high concentrations of apo-lactoferin. Infect Immun. 1976 Oct;14(4):911–918. doi: 10.1128/iai.14.4.911-918.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J. A., Segal W. Kinetics of Utilization of Organic Compounds in the Growth of Mycobacterium tuberculosis. J Bacteriol. 1965 Jul;90(1):157–163. doi: 10.1128/jb.90.1.157-163.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHARLEY P., ROSENSTEIN M., SHORE E., SALTMAN P. The role of chelation and binding equilibria in iron metabolism. Arch Biochem Biophys. 1960 Jun;88:222–226. doi: 10.1016/0003-9861(60)90226-5. [DOI] [PubMed] [Google Scholar]
- Dunn J. T., Spiro R. G. The alpha 2-macroglobulin of human plasma. I. Isolation and composition. J Biol Chem. 1967 Dec 10;242(23):5549–5555. [PubMed] [Google Scholar]
- FRANCIS J., MACTURK H. M., MADINAVEITIA J., SNOW G. A. Mycobactin, a growth factor for Mycobacterium johnei. I. Isolation from Mycobacterium phlei. Biochem J. 1953 Nov;55(4):596–607. doi: 10.1042/bj0550596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. Relationship between the tonB locus and iron transport in Escherichia coli. J Bacteriol. 1975 Nov;124(2):704–712. doi: 10.1128/jb.124.2.704-712.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. The inducible citrate-dependent iron transport system in Escherichia coli K12. Biochim Biophys Acta. 1973 Nov 30;330(1):90–101. doi: 10.1016/0005-2736(73)90287-3. [DOI] [PubMed] [Google Scholar]
- Griffiths E., Humphreys J. Bacteriostatic effect of human milk and bovine colostrum on Escherichia coli: importance of bicarbonate. Infect Immun. 1977 Feb;15(2):396–401. doi: 10.1128/iai.15.2.396-401.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C., Botham P. A. Pathways of glucose metabolism in Candida 107, a lipid-accumulating yeast. J Gen Microbiol. 1977 Oct;102(2):391–395. doi: 10.1099/00221287-102-2-391. [DOI] [PubMed] [Google Scholar]
- Ratledge C., Hall M. J. Influence of metal ions on the formation of mycobactin and salicylic acid in Mycobacterium smegmatis grown in static culture. J Bacteriol. 1971 Oct;108(1):314–319. doi: 10.1128/jb.108.1.314-319.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C., Macham L. P., Brown K. A., Marshall B. J. Iron transport in Mycobacterium smegmatis: a restricted role for salicylic acid in the extracellular environment. Biochim Biophys Acta. 1974 Nov 4;372(1):39–51. doi: 10.1016/0304-4165(74)90071-3. [DOI] [PubMed] [Google Scholar]
- SNOW G. A. THE STRUCTURE OF MYCOBACTIN P, A GROWTH FACTOR FOR MYCOBACTERIUM JOHNEI, AND THE SIGNIFICANCE OF ITS IRON COMPLEX. Biochem J. 1965 Jan;94:160–165. doi: 10.1042/bj0940160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar B. State of iron(3) in normal human serum: low molecular weight and protein ligands besides transferrin. Can J Biochem. 1970 Dec;48(12):1339–1350. doi: 10.1139/o70-208. [DOI] [PubMed] [Google Scholar]
- Stephenson M. C., Ratledge C. Iron transport in mycobacterium smegmatis: uptake of iron from Ferriexochelin. J Gen Microbiol. 1979 Jan;110(1):193–202. doi: 10.1099/00221287-110-1-193. [DOI] [PubMed] [Google Scholar]
- Wagegg W., Braun V. Ferric citrate transport in Escherichia coli requires outer membrane receptor protein fecA. J Bacteriol. 1981 Jan;145(1):156–163. doi: 10.1128/jb.145.1.156-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann G., Zähner H. Stoffwechselprodukte von Mikroorganismen. 115. Eisenaufnahme bei Neurospora crassa. I. Zur Spezifität des Eisentransportes. Arch Mikrobiol. 1973;88(1):49–60. [PubMed] [Google Scholar]



