Abstract
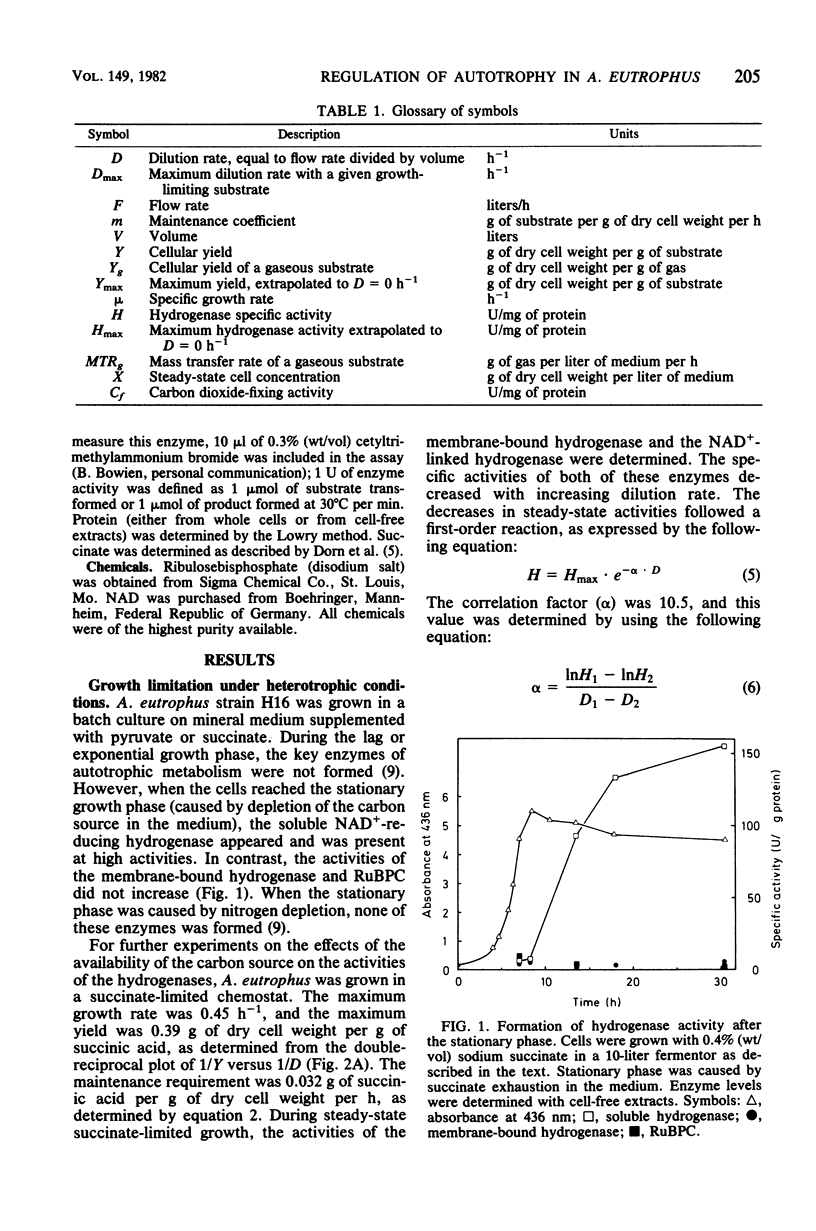
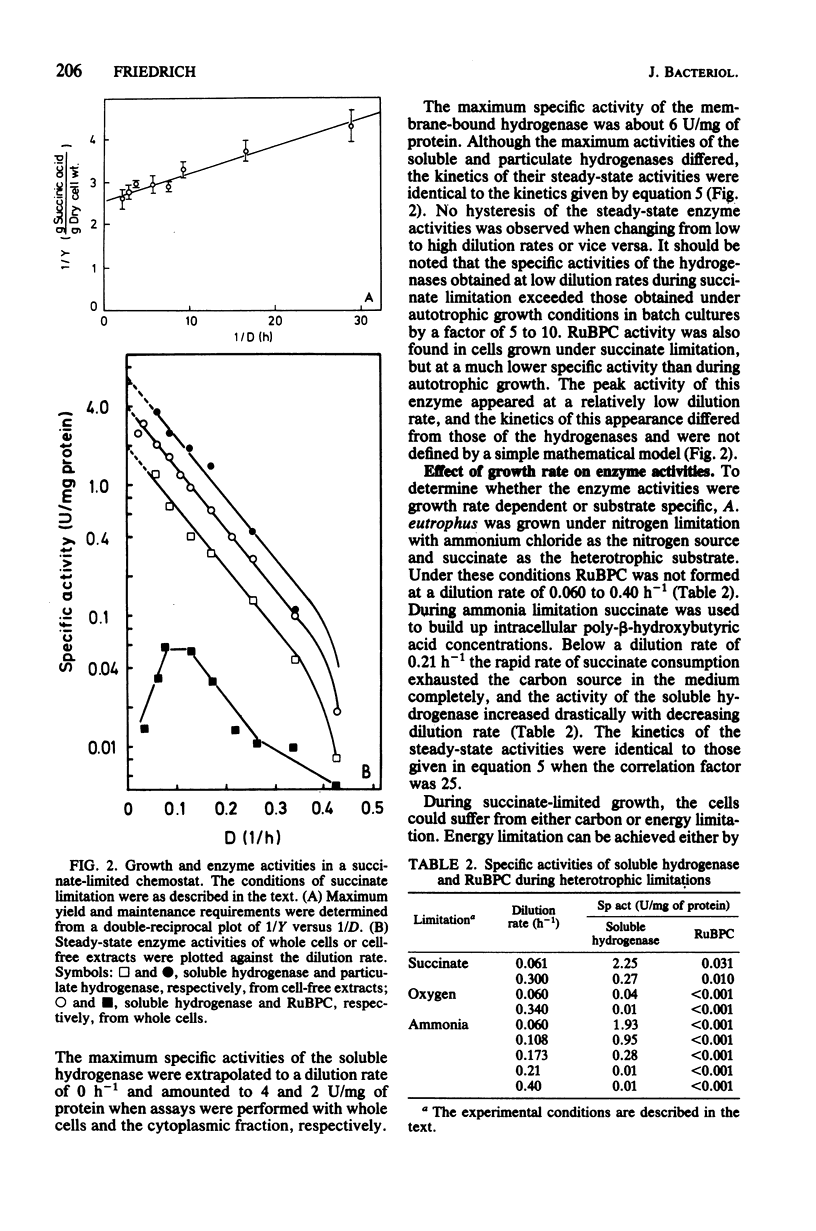
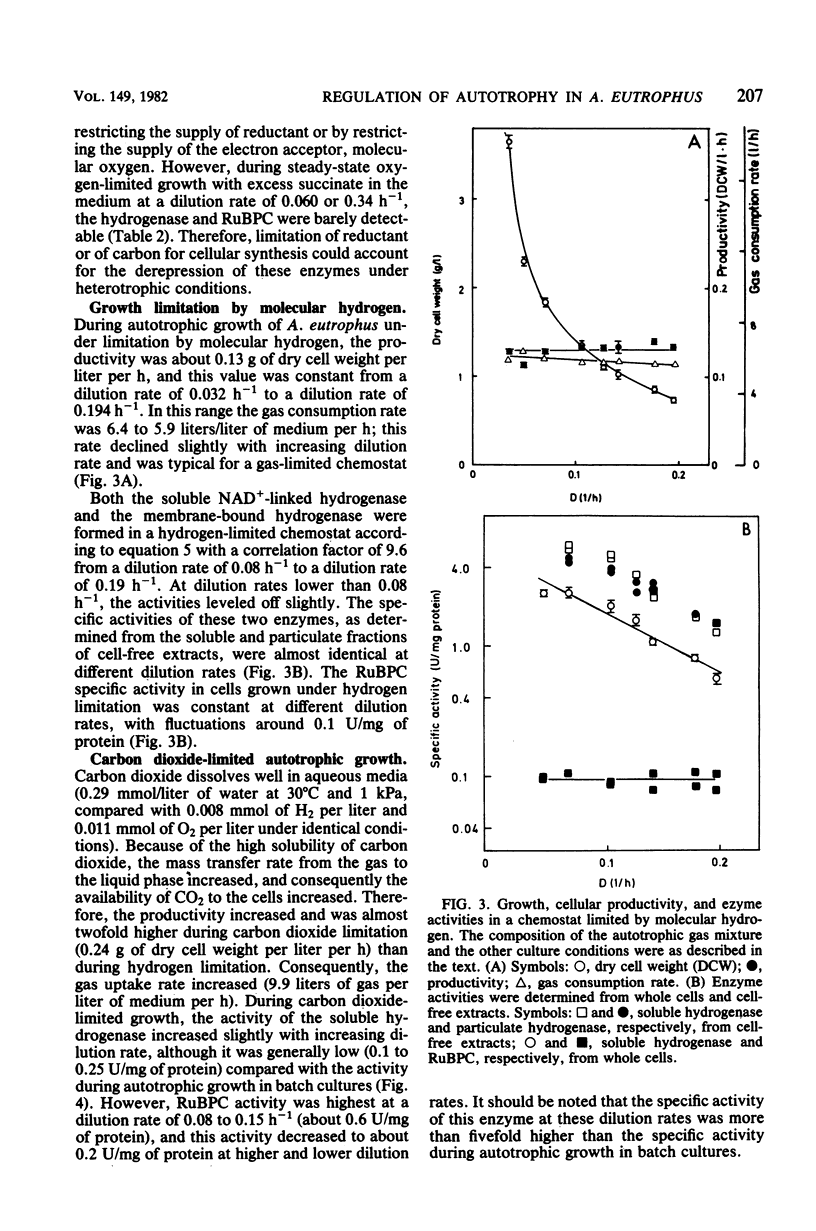
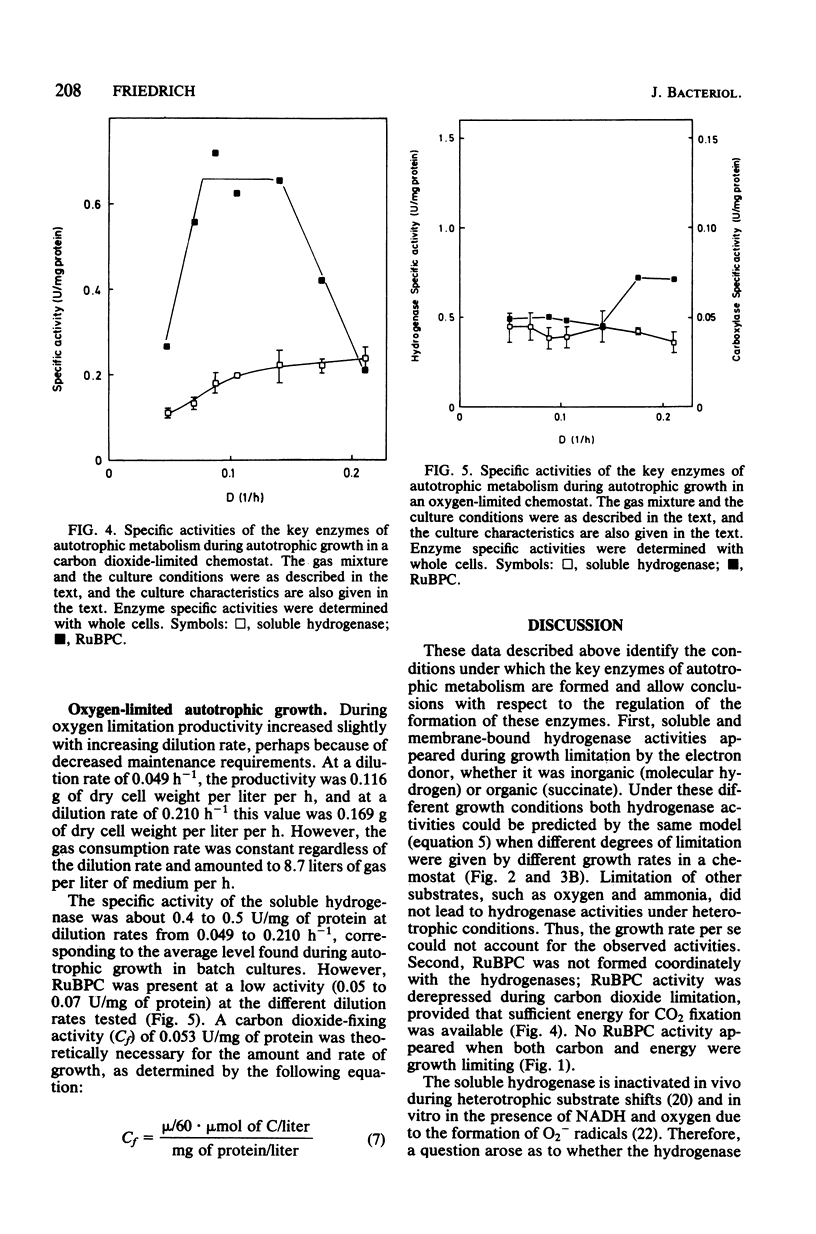
Alcaligenes eutrophus did not form the key enzymes of autotrophic metabolism, the soluble and particulate hydrogenases and ribulosebisphosphate carboxylase (RuBPC), during heterotrophic growth on succinate in batch cultures. During succinate-limited growth in a chemostat, high activities of both hydrogenases were observed. With decreasing dilution rate (D) the steady-state hydrogenase activity (H) followed first-order kinetics, expressed as follows: H = Hmax .e-alpha.D. An identical correlation was observed when autotrophic growth in a chemostat was limited by molecular hydrogen. During autotrophic growth under oxygen or carbon dioxide limitation, the activity if the soluble hydrogenase was low. These data suggested that hydrogenase formation depended on the availability of reducing equivalents to the cells. RuBPC activities were not correlated with the hydrogenase activities. During succinate-limited growth, RuBPC appeared at intermediate activities. During autotrophic growth in a carbon dioxide-limited chemostat, RuBPC was highly derepressed. RuBPC activity was not detected in cells that suffered from energy limitation with a surplus of carbon, as in a heterotrophic oxygen-limited chemostat, nor was it detected in cells limited in carbon and energy, as in the case of complete exhaustion of a heterotrophic substrate. From these data I concluded that RuBPC formation in A. eutrophus depends on two conditions, namely, carbon starvation and an excess of reducing equivalents.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowien B., Mayer F., Codd G. A., Schlegel H. G. Purification, some properties and quaternary structure of the D-ribulose 1,5-diphosphate carboxylase of Alcaligenes eutrophus. Arch Microbiol. 1976 Nov 2;110(23):157–166. doi: 10.1007/BF00690223. [DOI] [PubMed] [Google Scholar]
- Clarke P. H., Houldsworth M. A., Lilly M. D. Catabolite repression and the induction of amidase synthesis by Pseudomonas aeruginosa 8602 in continuous culture. J Gen Microbiol. 1968 Apr;51(2):225–234. doi: 10.1099/00221287-51-2-225. [DOI] [PubMed] [Google Scholar]
- Dorn M., Andreesen J. R., Gottschalk G. Fermentation of fumarate and L-malate by Clostridium formicoaceticum. J Bacteriol. 1978 Jan;133(1):26–32. doi: 10.1128/jb.133.1.26-32.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger R. F., Deeley R. G., Mullinix K. P. Regulation of gene expression in prokaryotic organisms. Adv Genet. 1976;18:1–67. doi: 10.1016/s0065-2660(08)60436-8. [DOI] [PubMed] [Google Scholar]
- Jensen D. E., Neidhardt F. C. Effect of growth rate on histidine catabolism and histidase synthesis in Aerobacter aerogenes. J Bacteriol. 1969 Apr;98(1):131–142. doi: 10.1128/jb.98.1.131-142.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn G. D., McFadden B. A. Factors affecting the synthesis and degradation of ribulose-1,5-diphosphate carboxylase in Hydrogenomonas facilis and Hydrogenomonas eutropha. J Bacteriol. 1968 Mar;95(3):937–946. doi: 10.1128/jb.95.3.937-946.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Hanus F. J., Evans H. J. Regulation of hydrogenase in Rhizobium japonicum. J Bacteriol. 1979 Feb;137(2):825–829. doi: 10.1128/jb.137.2.825-829.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Gottschal J. C. Influence of dilution rate on NAD(P) and NAD(P)H concentrations and ratios in a Pseudomonas sp. grown in continuous culture. J Gen Microbiol. 1976 Jun;94(2):333–341. doi: 10.1099/00221287-94-2-333. [DOI] [PubMed] [Google Scholar]
- Matin A., Grootjans A., Hogenhuis H. Influence of dilution rate on enzymes of intermediary metabolism in two freshwater bacteria grown in continuous culture. J Gen Microbiol. 1976 Jun;94(2):323–332. doi: 10.1099/00221287-94-2-323. [DOI] [PubMed] [Google Scholar]
- Pirt S. J. The maintenance energy of bacteria in growing cultures. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. Hydrogen metabolism in aerobic hydrogen-oxidizing bacteria. Biochimie. 1978;60(3):297–305. doi: 10.1016/s0300-9084(78)80826-8. [DOI] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G. Production of superoxide radicals by soluble hydrogenase from Alcaligenes eutrophus H16. Biochem J. 1981 Jan 1;193(1):99–107. doi: 10.1042/bj1930099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Influence of environment on the content and composition of microbial free amino acid pools. J Gen Microbiol. 1970 Dec;64(2):171–185. doi: 10.1099/00221287-64-2-171. [DOI] [PubMed] [Google Scholar]


