Abstract
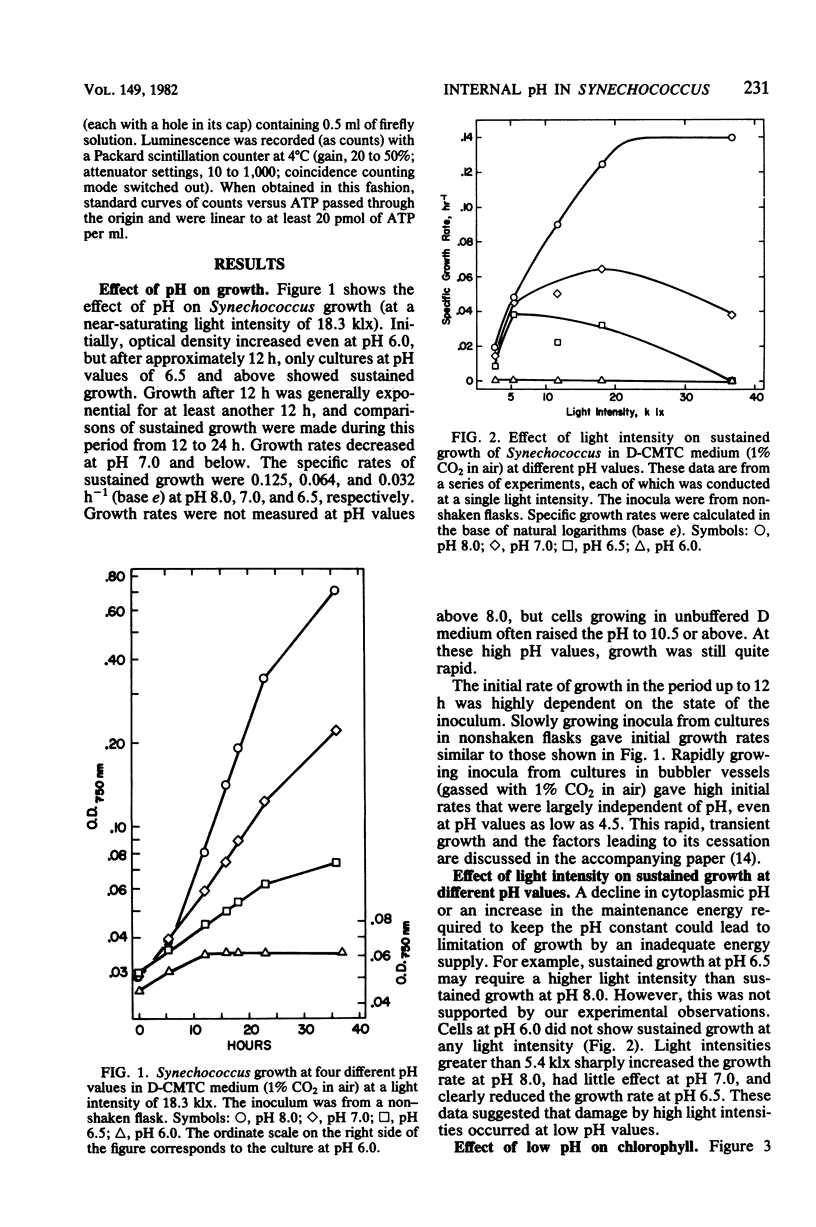
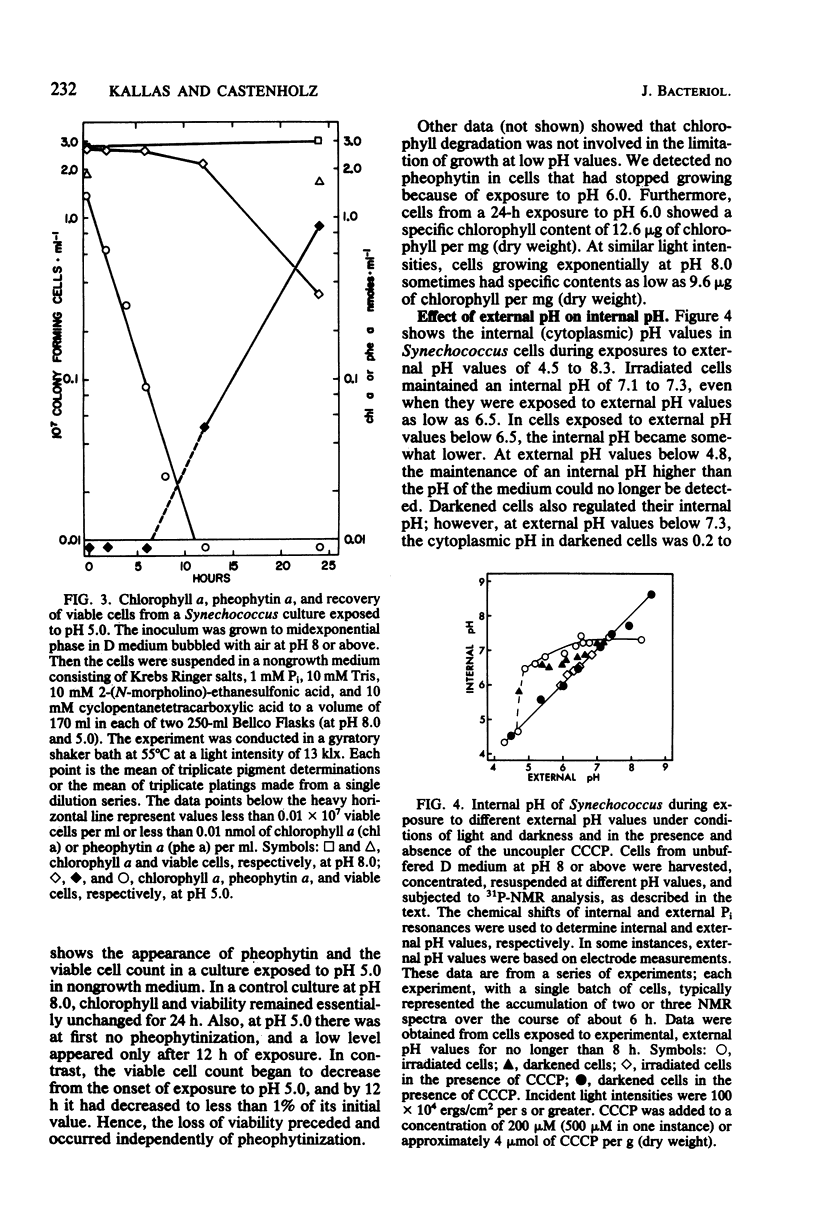
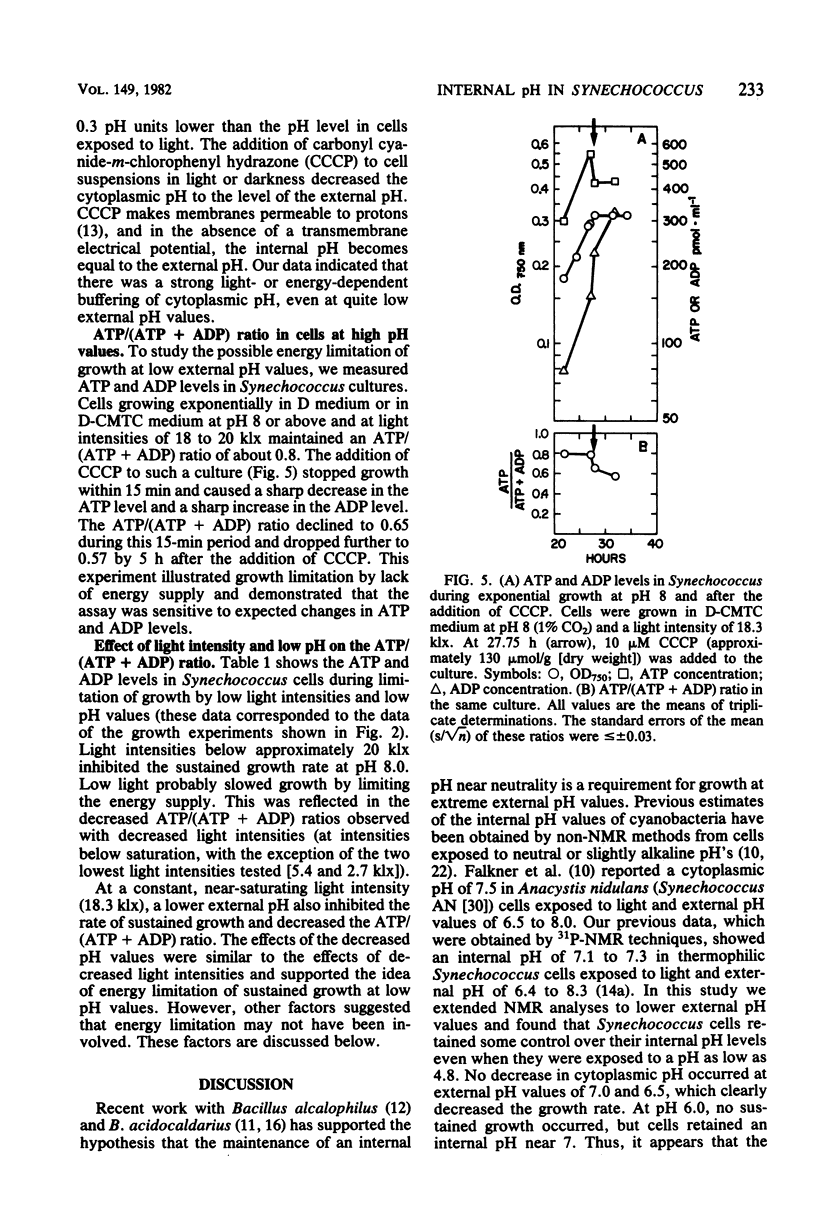
Y-7c-s Synechococcus thermophilic strain grew at its maximum rate at pH 8 and above. The growth rate of this strain was inhibited at pH 7.0 and below, and at pH 6.0 there was no sustained growth. At a suboptimal pH, high light intensity further depressed the growth rate. The inhibition of growth resulted neither from pheophytinization nor from a low chlorophyll content. At pH 5.0 a loss of viability preceded the appearance of pheophytin. Cells exposed to low, growth-inhibiting external pH levels continued to maintain a high internal pH (pH 7.1 to 7.3, as determined at moderate light intensities by 31P nuclear magnetic resonance spectroscopy). Even during exposure to pH 4.8, cells retained a relatively high internal pH. Thus, it appeared that the inhibition of growth at low pH was not caused by acidification of the cytoplasm. Darkened cells maintained a slightly lower internal pH than irradiated cells. The ATP/(ATP + ADP) ratio decreased from 0.80 to 0.82 at pH 8.0 to about 0.6 when growth was limited by exposure to pH 6.0 or by low light intensity. It is possible, but not likely, that a limitation of the energy supply may slow or stop growth when the external pH is lowered.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeliovich A., Shilo M. Photooxidative death in blue-green algae. J Bacteriol. 1972 Sep;111(3):682–689. doi: 10.1128/jb.111.3.682-689.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley P. J., Stewart W. D. ATP pools and transientss in the blue-green alga, Anabaena cylindrica. Arch Microbiol. 1976 Jul;108(3):249–258. doi: 10.1007/BF00454849. [DOI] [PubMed] [Google Scholar]
- Brock T. D. Lower pH limit for the existence of blue-green algae: evolutionary and ecological implications. Science. 1973 Feb 2;179(4072):480–483. doi: 10.1126/science.179.4072.480. [DOI] [PubMed] [Google Scholar]
- Castenholz R. W. Thermophilic blue-green algae and the thermal environment. Bacteriol Rev. 1969 Dec;33(4):476–504. doi: 10.1128/br.33.4.476-504.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. G., Atkinson D. E. Adenine nucleotide concentrations and turnover rates. Their correlation with biological activity in bacteria and yeast. Adv Microb Physiol. 1977;15:253–306. doi: 10.1016/s0065-2911(08)60318-5. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F. The cyanobacterial genome, its expression, and the control of that expression. Adv Microb Physiol. 1979;20:1–102. doi: 10.1016/s0065-2911(08)60206-4. [DOI] [PubMed] [Google Scholar]
- Eloff J. N., Steinitz Y., Shilo M. Photooxidation of cyanobacteria in natural conditions. Appl Environ Microbiol. 1976 Jan;31(1):119–126. doi: 10.1128/aem.31.1.119-126.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffanti A. A., Davidson L. F., Mann T. M., Krulwich T. A. Nigericin-induced death of an acidophilic bacterium. J Gen Microbiol. 1979 Sep;114(1):201–206. doi: 10.1099/00221287-114-1-201. [DOI] [PubMed] [Google Scholar]
- Guffanti A. A., Susman P., Blanco R., Krulwich T. A. The protonmotive force and alpha-aminoisobutyric acid transport in an obligately alkalophilic bacterium. J Biol Chem. 1978 Feb 10;253(3):708–715. [PubMed] [Google Scholar]
- Hopfer U., Lehninger A. L., Thompson T. E. Protonic conductance across phospholipid bilayer membranes induced by uncoupling agents for oxidative phosphorylation. Proc Natl Acad Sci U S A. 1968 Feb;59(2):484–490. doi: 10.1073/pnas.59.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallas T., Castenholz R. W. Rapid transient growth at low pH in the cyanobacterium Synechococcus sp. J Bacteriol. 1982 Jan;149(1):237–246. doi: 10.1128/jb.149.1.237-246.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallas T., Dahlquist F. W. Phosphorus-31 nuclear magnetic resonance analysis of internal pH during photosynthesis in the cyanobacterium Synechococcus. Biochemistry. 1981 Sep 29;20(20):5900–5907. doi: 10.1021/bi00523a038. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Unemoto T. Streptococcus faecalis mutants defective in regulation of cytoplasmic pH. J Bacteriol. 1980 Sep;143(3):1187–1193. doi: 10.1128/jb.143.3.1187-1193.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Davidson L. F., Filip S. J., Jr, Zuckerman R. S., Guffanti A. A. The protonmotive force and beta-galactoside transport in Bacillus acidocaldarius. J Biol Chem. 1978 Jul 10;253(13):4599–4603. [PubMed] [Google Scholar]
- Lundin A., Thore A. Analytical information obtainable by evaluation of the time course of firefly bioluminescence in the assay of ATP. Anal Biochem. 1975 May 26;66(1):47–63. doi: 10.1016/0003-2697(75)90723-x. [DOI] [PubMed] [Google Scholar]
- Lundin A., Thore A. Comparison of methods for extraction of bacterial adenine nucleotides determined by firefly assay. Appl Microbiol. 1975 Nov;30(5):713–721. doi: 10.1128/am.30.5.713-721.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K., Nishimura M. Estimation of internal pH in cells of blue-green algae in the dark and under illumination. J Biochem. 1977 Aug;82(2):483–487. [PubMed] [Google Scholar]
- Meeks J. C., Castenholz R. W. Growth and photosynthesis in an extreme thermophile, Synechococcus lividus (Cyanophyta). Arch Mikrobiol. 1971;78(1):25–41. doi: 10.1007/BF00409086. [DOI] [PubMed] [Google Scholar]
- Padan E., Schuldiner S. Energy transduction in the photosynthetic membranes of the cyanobacterium (blue-green alga) P-lectonema boryanum. J Biol Chem. 1978 May 10;253(9):3281–3286. [PubMed] [Google Scholar]
- Pfennig N. Rhodopseudomonas acidophila, sp. n., a new species of the budding purple nonsulfur bacteria. J Bacteriol. 1969 Aug;99(2):597–602. doi: 10.1128/jb.99.2.597-602.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick U., Rottenberg H., Avron M. The dependence of photophosphorylation in chloroplasts on delta pH and external pH. FEBS Lett. 1974 Nov 1;48(1):32–36. doi: 10.1016/0014-5793(74)81055-0. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of transmembrane electrochemical proton gradients. J Bioenerg. 1975 May;7(2):61–74. doi: 10.1007/BF01558427. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Cohen-Bazire G. Phototrophic prokaryotes: the cyanobacteria. Annu Rev Microbiol. 1977;31:225–274. doi: 10.1146/annurev.mi.31.100177.001301. [DOI] [PubMed] [Google Scholar]



