Abstract
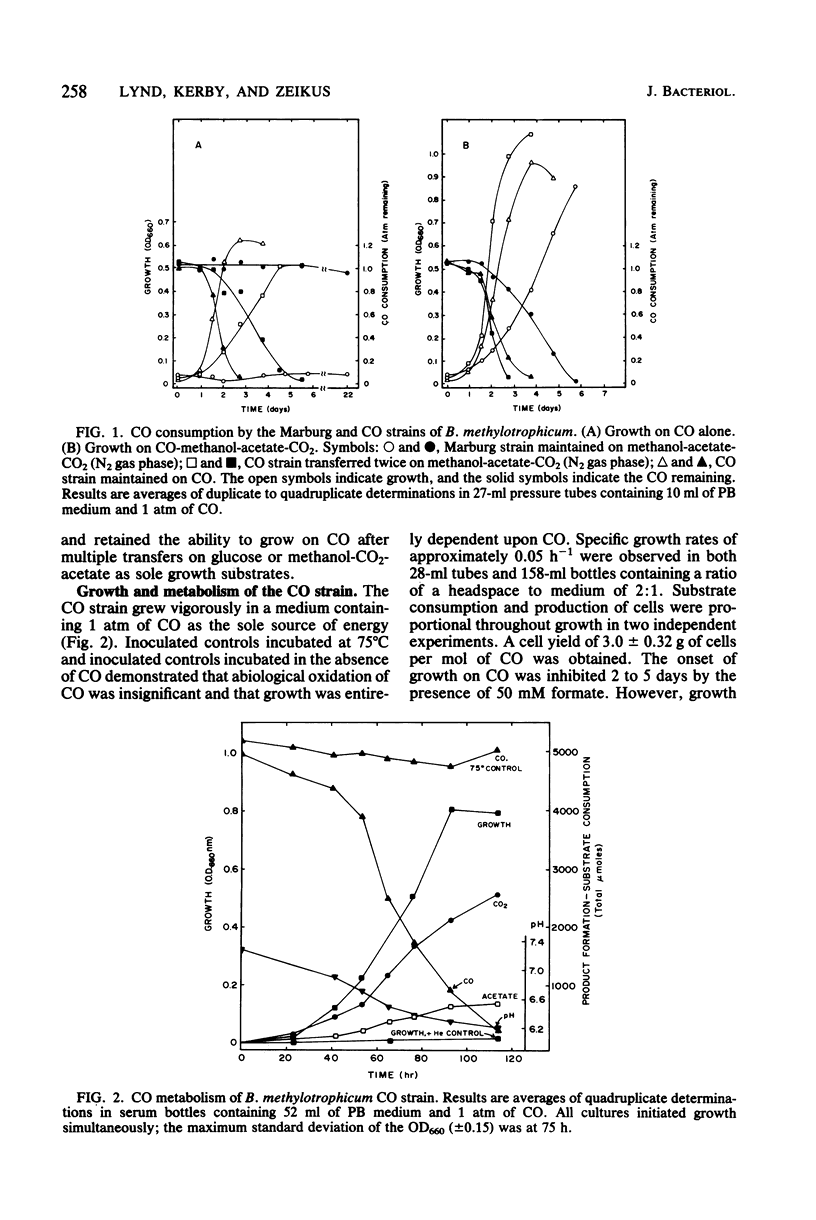
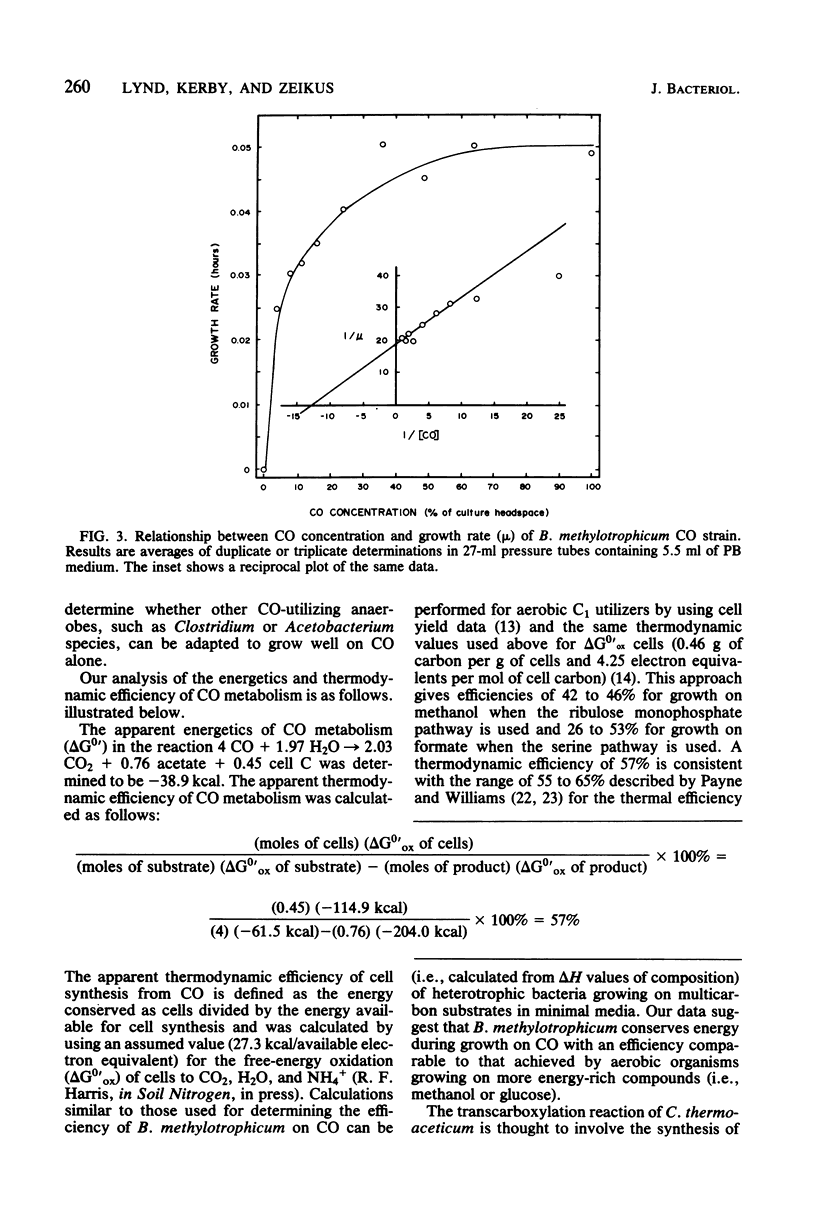
The Marburg strain of Butyribacterium methylotrophicum did not grow on CO alone but did consume CO during growth on a variety of substrates in the presence of a 100% CO gas phase. We selected a strain (the CO strain) that grew vigorously on CO alone. The ability of the CO strain to grow on CO was stable through multiple transfers in the absence of CO. CO dehydrogenase activity was lower in the CO strain grown on CO (13.3 micromol/min per mg of protein) than in the Marburg strain grown on methanol-acetate (47.2 mumol/min per mg of protein); thus, the levels of this enzyme did not explain the growth on CO. CO was dissimilated to acetate and CO2 with the following stoichiometry: 4 CO leads to 2.17 CO2 + 0.74 acetate. We observed a growth rate of 0.05 h-1, a final optical density at 660 nm of 0.8, and a cell yield of 3.0 g of cells per mol of CO during growth of the CO strain. Growing cultures of the CO strain displayed a Ks for CO of 28 to 56 microM. The apparent thermodynamic efficiency of cell synthesis from CO was 57%. Energetic and biochemical aspects of CO metabolism are described.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colby J., Dalton H., Whittenbury R. Biological and biochemical aspects of microbial growth on C1 compounds. Annu Rev Microbiol. 1979;33:481–517. doi: 10.1146/annurev.mi.33.100179.002405. [DOI] [PubMed] [Google Scholar]
- Daniels L., Fuchs G., Thauer R. K., Zeikus J. G. Carbon monoxide oxidation by methanogenic bacteria. J Bacteriol. 1977 Oct;132(1):118–126. doi: 10.1128/jb.132.1.118-126.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekert G. B., Thauer R. K. Carbon monoxide oxidation by Clostridium thermoaceticum and Clostridium formicoaceticum. J Bacteriol. 1978 Nov;136(2):597–606. doi: 10.1128/jb.136.2.597-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake H. L., Hu S. I., Wood H. G. Purification of carbon monoxide dehydrogenase, a nickel enzyme from Clostridium thermocaceticum. J Biol Chem. 1980 Aug 10;255(15):7174–7180. [PubMed] [Google Scholar]
- Ferenci T. Carbon monoxide-stimulated respiration in methane-utilizing bacteria. FEBS Lett. 1974 Apr 15;41(1):94–98. doi: 10.1016/0014-5793(74)80962-2. [DOI] [PubMed] [Google Scholar]
- Fuchs G., Schnitker U., Thauer R. K. Carbon monoxide oxidation by growing cultures of Clostridium pasteurianum. Eur J Biochem. 1974 Nov 1;49(1):111–115. doi: 10.1111/j.1432-1033.1974.tb03816.x. [DOI] [PubMed] [Google Scholar]
- Goldberg I., Rock J. S., Ben-Bassat A., Mateles R. I. Bacterial yields on methanol, methylamine, formaldehyde, and formate. Biotechnol Bioeng. 1976 Dec;18(12):1657–1668. doi: 10.1002/bit.260181202. [DOI] [PubMed] [Google Scholar]
- Harris R. F., Adams S. S. Determination of the carbon-bound electron composition of microbial cells and metabolites by dichromate oxidation. Appl Environ Microbiol. 1979 Feb;37(2):237–243. doi: 10.1128/aem.37.2.237-243.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkconnell S., Hegeman G. D. Mechanism of oxidation of carbon monoxide by bacteria. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1584–1587. doi: 10.1016/0006-291x(78)91402-x. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G. Total synthesis of acetate from CO2 by heterotrophic bacteria. Annu Rev Microbiol. 1969;23:515–538. doi: 10.1146/annurev.mi.23.100169.002503. [DOI] [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Carbon monoxide:methylene blue oxidoreductase from Pseudomonas carboxydovorans. J Bacteriol. 1980 Jan;141(1):74–80. doi: 10.1128/jb.141.1.74-80.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J. Energy yields and growth of heterotrophs. Annu Rev Microbiol. 1970;24:17–52. doi: 10.1146/annurev.mi.24.100170.000313. [DOI] [PubMed] [Google Scholar]
- Payne W. J., Williams M. L. Carbon assimilation from simple and complex media by prtotrophic heterotrophic bacteria. Biotechnol Bioeng. 1976 Nov;18(11):1653–1655. doi: 10.1002/bit.260181117. [DOI] [PubMed] [Google Scholar]
- Schulman M., Ghambeer R. K., Ljungdahl L. G., Wood H. G. Total synthesis of acetate from CO2. VII. Evidence with Clostridium thermoaceticum that the carboxyl of acetate is derived from the carboxyl of pyruvate by transcarboxylation and not by fixation of CO2. J Biol Chem. 1973 Sep 25;248(18):6255–6261. [PubMed] [Google Scholar]
- Thauer R. K., Fuchs G., Käufer B., Schnitker U. Carbon-monoxide oxidation in cell-free extracts of Clostridium pasteurianum. Eur J Biochem. 1974 Jun 15;45(2):343–349. doi: 10.1111/j.1432-1033.1974.tb03559.x. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L. Anaerobic growth of a Rhodopseudomonas species in the dark with carbon monoxide as sole carbon and energy substrate. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3298–3302. doi: 10.1073/pnas.73.9.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welty F. K., Wood H. G. Purification of the "corrinoid" enzyme involved in the synthesis of acetate by Clostridium thermoaceticum. J Biol Chem. 1978 Aug 25;253(16):5832–5838. [PubMed] [Google Scholar]
- Zeikus J. G. Chemical and fuel production by anaerobic bacteria. Annu Rev Microbiol. 1980;34:423–464. doi: 10.1146/annurev.mi.34.100180.002231. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Fuchs G., Kenealy W., Thauer R. K. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol. 1977 Nov;132(2):604–613. doi: 10.1128/jb.132.2.604-613.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



