Abstract
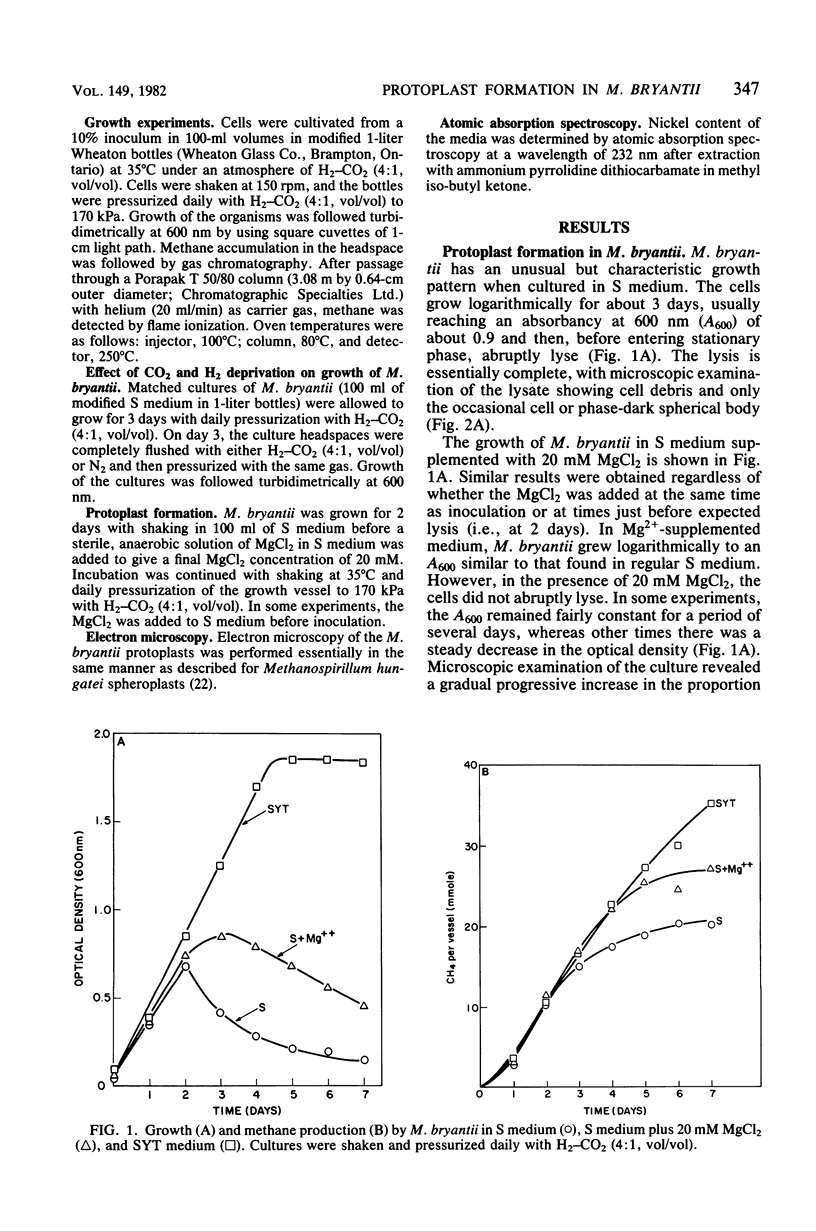
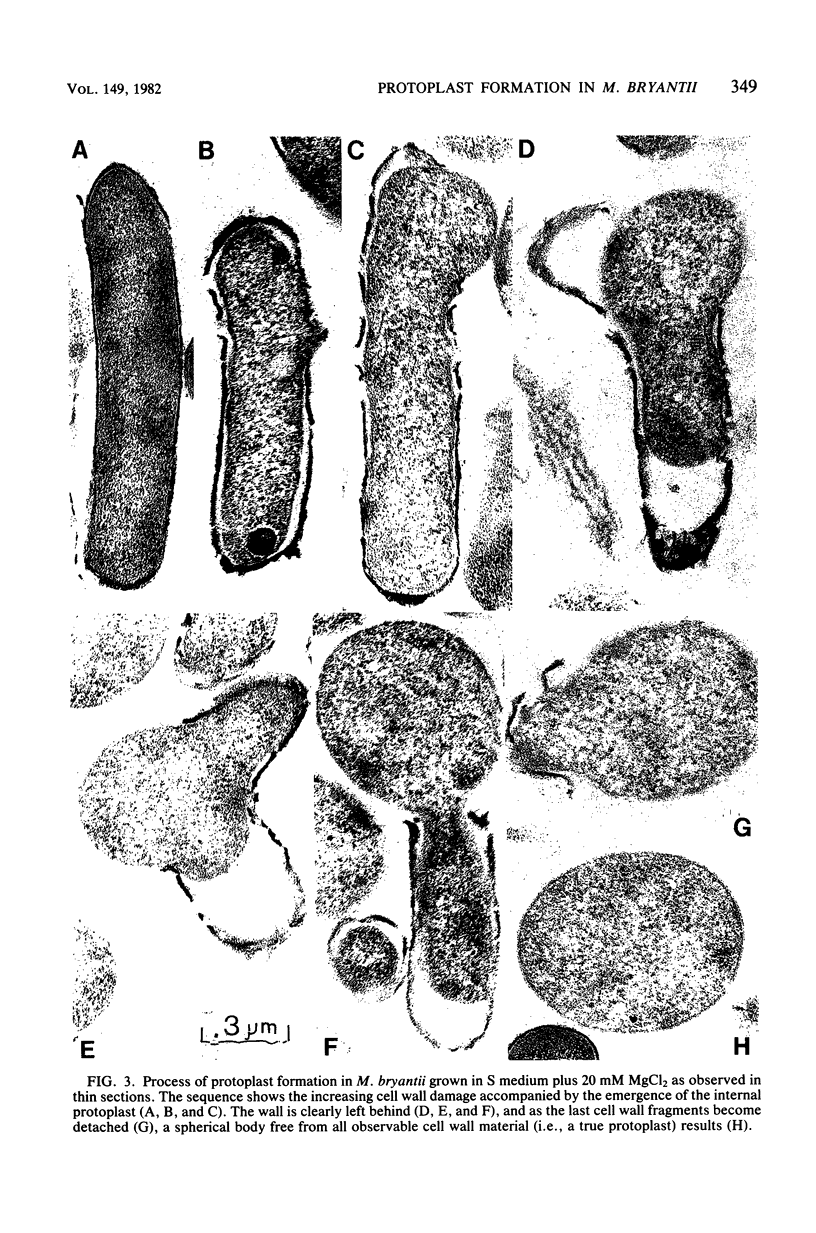
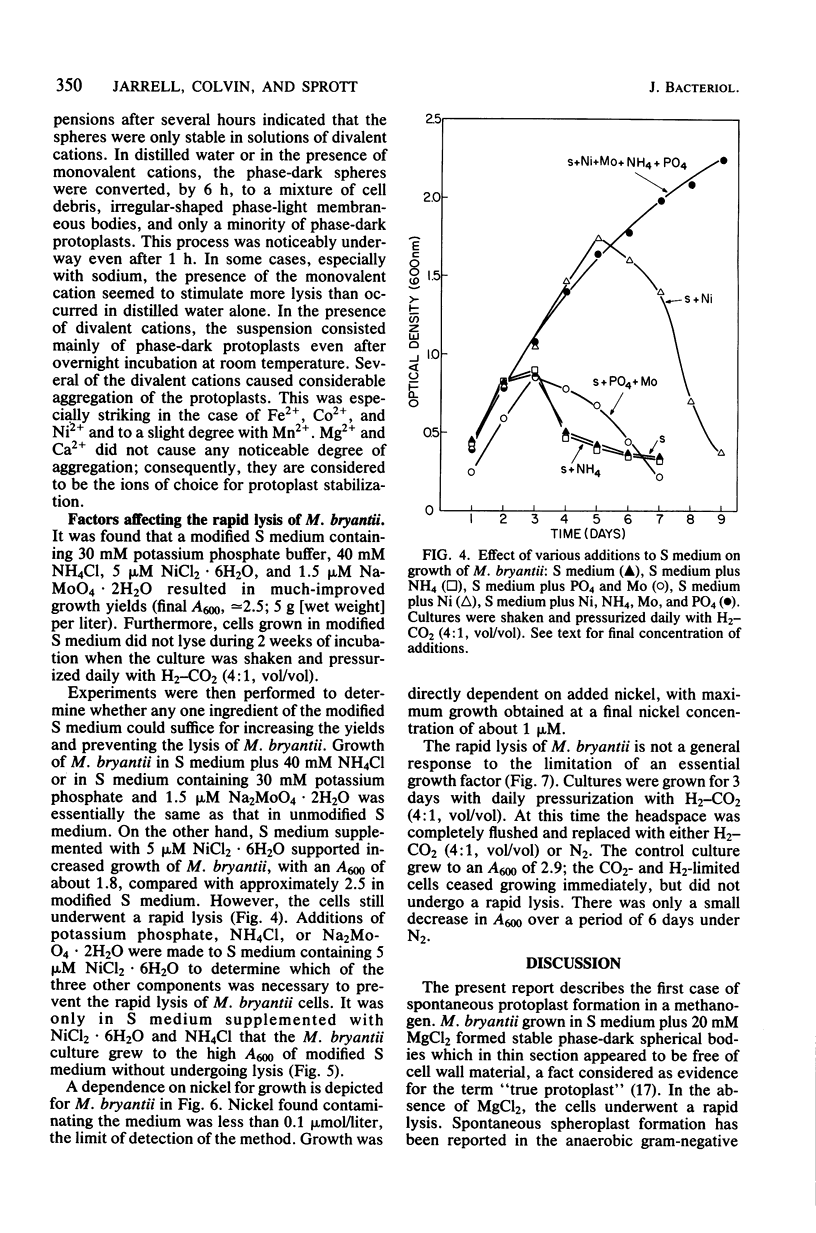
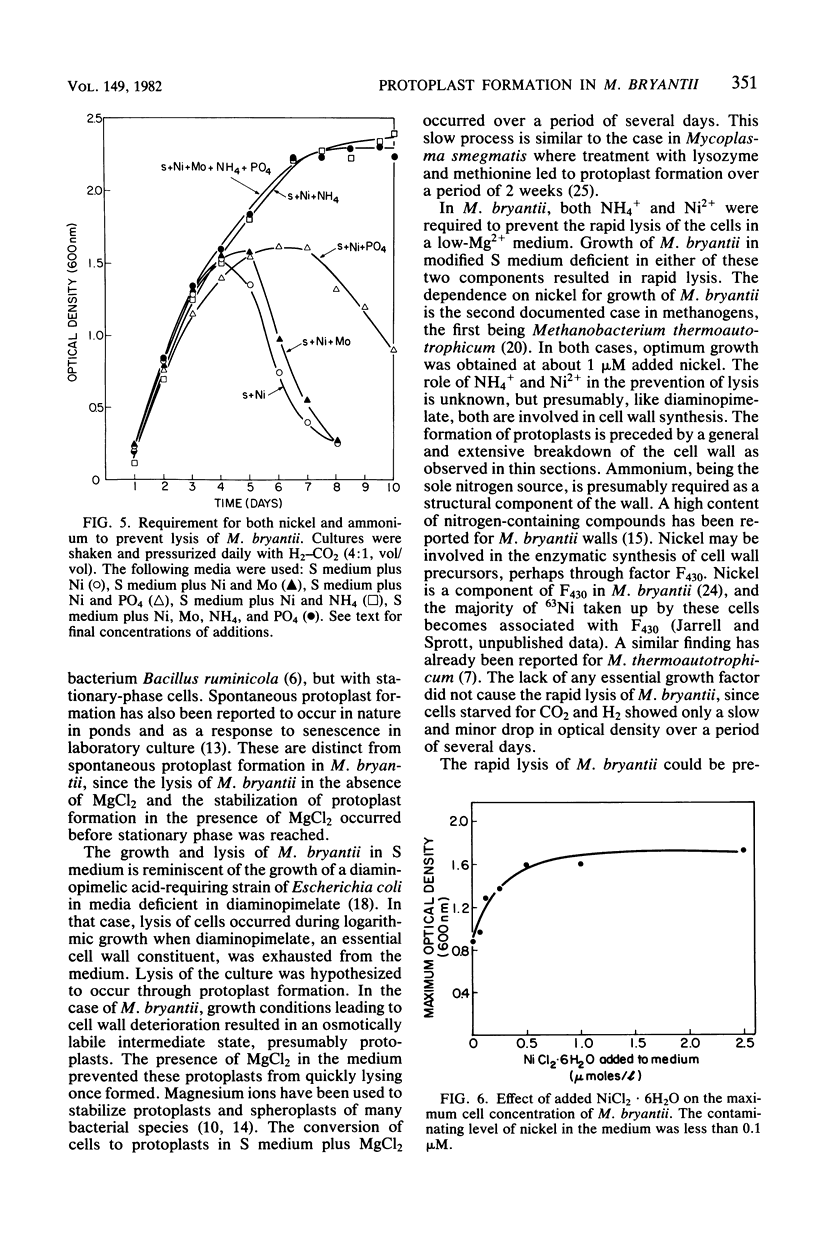
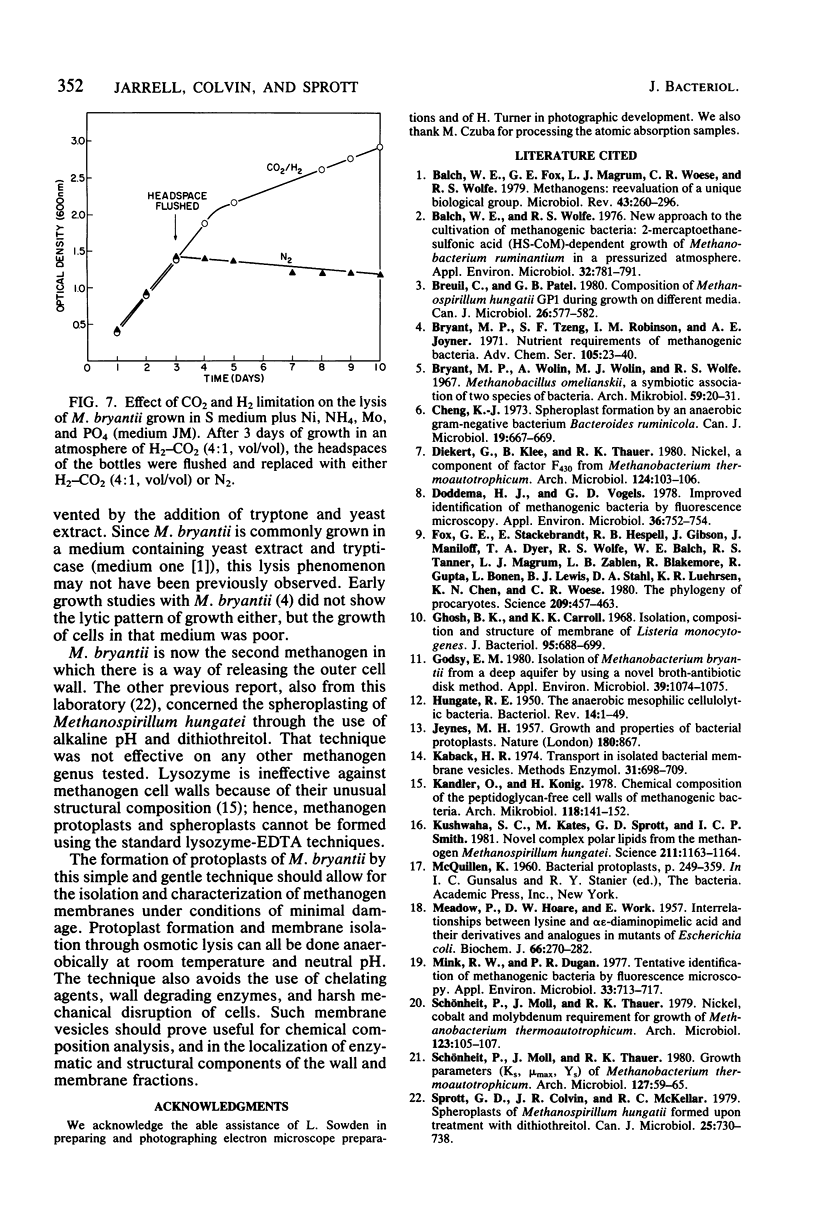
Methanobacterium bryantii was found to undergo rapid lysis when grown in a prereduced chemically defined medium under H2-CO2 (4:1, vol/vol). The addition of 20 mM MgCl2 to the medium gave, rather than rapid lysis, a gradual formation of phase-dark spherical bodies which in thin section appeared as true protoplasts. In general, the protoplasts were stabilized by divalent but not monovalent cations and, unlike whole cells, were sensitive to lysis by Triton X-100. Electron microscopic examination revealed that protoplast formation was preceded by a general breakdown of the cell wall with an apparent squeezing out of the protoplast through the degraded wall. The growth of cells was greatly increased and not accompanied by detectable lysis in a medium modified by elevating the levels of nickel and ammonium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuil C., Patel G. B. Composition of Methanospirillum hungatii GP1 during growth on different media. Can J Microbiol. 1980 May;26(5):577–582. doi: 10.1139/m80-102. [DOI] [PubMed] [Google Scholar]
- Bryant M. P., Wolin E. A., Wolin M. J., Wolfe R. S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Mikrobiol. 1967;59(1):20–31. doi: 10.1007/BF00406313. [DOI] [PubMed] [Google Scholar]
- Cheng K. J. Spheroplast formation by an anaerobic gram-negative bacterium Bacteroides ruminicola. Can J Microbiol. 1973 May;19(5):667–669. doi: 10.1139/m73-108. [DOI] [PubMed] [Google Scholar]
- Diekert G., Klee B., Thauer R. K. Nickel, a component of factor F430 from Methanobacterium thermoautotrophicum. Arch Microbiol. 1980 Jan;124(1):103–106. doi: 10.1007/BF00407036. [DOI] [PubMed] [Google Scholar]
- Doddema H. J., Vogels G. D. Improved identification of methanogenic bacteria by fluorescence microscopy. Appl Environ Microbiol. 1978 Nov;36(5):752–754. doi: 10.1128/aem.36.5.752-754.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Ghosh B. K., Carroll K. K. Isolation, composition, and structure of membrane of Listeria monocytogenes. J Bacteriol. 1968 Feb;95(2):688–699. doi: 10.1128/jb.95.2.688-699.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsy E. M. Isolation of Methanobacterium bryantii from a Deep Aquifer by Using a Novel Broth-Antibiotic Disk Method. Appl Environ Microbiol. 1980 May;39(5):1074–1075. doi: 10.1128/aem.39.5.1074-1075.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Transport in isolated bacterial membrane vesicles. Methods Enzymol. 1974;31:698–709. doi: 10.1016/0076-6879(74)31075-0. [DOI] [PubMed] [Google Scholar]
- Kandler O., König H. Chemical composition of the peptidoglycan-free cell walls of methanogenic bacteria. Arch Microbiol. 1978 Aug 1;118(2):141–152. doi: 10.1007/BF00415722. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Sprott G. D., Smith I. C. Novel complex polar lipids from the methanogenic archaebacterium Methanospirillum hungatei. Science. 1981 Mar 13;211(4487):1163–1164. doi: 10.1126/science.7466385. [DOI] [PubMed] [Google Scholar]
- MEADOW P., HOARE D. S., WORK E. Interrelationships between lysine and alpha epsilon-diaminopimelic acid and their derivatives and analogues in mutants of Escherichia coli. Biochem J. 1957 Jun;66(2):270–282. doi: 10.1042/bj0660270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink R. W., Dugan P. R. Tentative identification of methanogenic bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 Mar;33(3):713–717. doi: 10.1128/aem.33.3.713-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönheit P., Moll J., Thauer R. K. Nickel, cobalt, and molybdenum requirement for growth of Methanobacterium thermoautotrophicum. Arch Microbiol. 1979 Oct;123(1):105–107. doi: 10.1007/BF00403508. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., Colvin J. R., McKellar R. C. Spheroplasts of Methanospirillum hungatii formed upon treatment with dithiothreitol. Can J Microbiol. 1979 Jun;25(6):730–738. doi: 10.1139/m79-106. [DOI] [PubMed] [Google Scholar]
- Ward T. E., Frea J. I. Sediment distribution of methanogenic bacteria in lake erie and cleveland harbor. Appl Environ Microbiol. 1980 Mar;39(3):597–603. doi: 10.1128/aem.39.3.597-603.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman W. B., Wolfe R. S. Presence of nickel in factor F430 from Methanobacterium bryantii. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1196–1201. doi: 10.1016/0006-291x(80)90413-1. [DOI] [PubMed] [Google Scholar]
- Yabu K., Takahashi S. Protoplast formation of selected Mycobacterium smegmatis mutants by lysozyme in combination with methionine. J Bacteriol. 1977 Mar;129(3):1628–1631. doi: 10.1128/jb.129.3.1628-1631.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]