Abstract
The manner in which the thousands of synaptic inputs received by a pyramidal neuron are summed is critical both to our understanding of the computations that may be performed by single neurons and of the codes used by neurons to transmit information. Recent work on pyramidal cell dendrites has shown that subthreshold synaptic inputs are modulated by voltage-dependent channels, raising the possibility that summation of synaptic responses is influenced by the active properties of dendrites. Here, we use somatic and dendritic whole-cell recordings to show that pyramidal cells in hippocampal area CA3 sum distal and proximal excitatory postsynaptic potentials sublinearly and actively, that the degree of nonlinearity depends on the magnitude and timing of the excitatory postsynaptic potentials, and that blockade of transient potassium channels linearizes summation. Nonlinear summation of synaptic inputs could have important implications for the computations performed by single neurons and also for the role of the mossy fiber and perforant path inputs to hippocampal area CA3.
A question central to our understanding of synaptic integration is “How does a neuron summate the tens of thousands of synaptic inputs that it receives, hundreds of which may be active in a period of a few milliseconds?” This question has two related components. First, are active processes such as activation of voltage-dependent channels involved in summation of voltage changes? Second, what mathematical functions are computed by neurons receiving multiple nearly simultaneous inputs?
Synaptic integration has been considered to be governed by the passive properties of the neuron. In most cases, passive models predict linear summation of synaptic inputs. That is, the response to two or more simultaneous inputs should be the arithmetic sum of the responses to those same inputs elicited separately. However, passive summation may be sublinear (1, 2) if either (i) the magnitude of the voltage change caused by one input is sufficient to alter the driving force at the other synapse or (ii) the conductance change resulting from the opening of synaptic channels decreases the neuron’s input resistance, leading to shunting of synaptic current. This model of passive synaptic integration makes important predictions about the computational function of neurons and their dendrites (2–4), as well as about the kinds of neural codes that single neurons might be able to interpret (5).
Recent work has demonstrated that subthreshold synaptic inputs activate voltage-dependent sodium and calcium channels in the soma and dendrites of pyramidal cells (6, 7), suggesting that synaptic integration may be an active process rather than being governed solely by the passive properties of neurons. Theoretical studies have suggested that the activation of sodium or calcium channels may provide a mechanism for supralinear summation of synaptic inputs, thereby allowing dendritic branches to perform nonlinear computations or to detect coincident inputs (3, 8).
However, pyramidal cell dendrites contain a high density of voltage-dependent potassium channels, which attenuate the propagation of excitatory postsynaptic potentials (EPSPs) and action potentials in dendrites (9). These channels may act to counter or modulate the actions of dendritic sodium and calcium channels. Thus, the net effect of somato-dendritic voltage-dependent channels on the summation of synaptic inputs is likely to depend on the amplitude, time course, and spatial location of the voltage changes (7, 10), as well as on the density and distribution of the various channel types.
To assess the properties and mechanisms of EPSP summation in pyramidal cells, we took advantage of our ability to stimulate independently two afferent inputs to pyramidal neurons in hippocampal area CA3. Cells in layer II of the entorhinal cortex (EC) form excitatory synapses on the distal apical dendrites of CA3 pyramidal cells via their perforant path axons (11, 12). Layer II cells also project disynaptically to CA3 via the dentate granule cells which send their mossy fiber axons to the proximal apical dendrites of CA3 pyramidal cells (Fig. 1A). Thus, CA3 pyramidal cells receive convergent excitatory synaptic inputs from the EC via these two pathways (13). Theoretical studies of the hippocampus have suggested that the facilitation of Hebbian synaptic plasticity by convergent mossy fiber and perforant path EPSPs is an important functional feature of hippocampal circuitry (14–16). Our understanding of the propagation of activity from EC to the hippocampus and also of the role of these pathways in hippocampal function therefore depends on an understanding of how these proximal and distal EPSPs are summed in CA3 pyramidal cells.
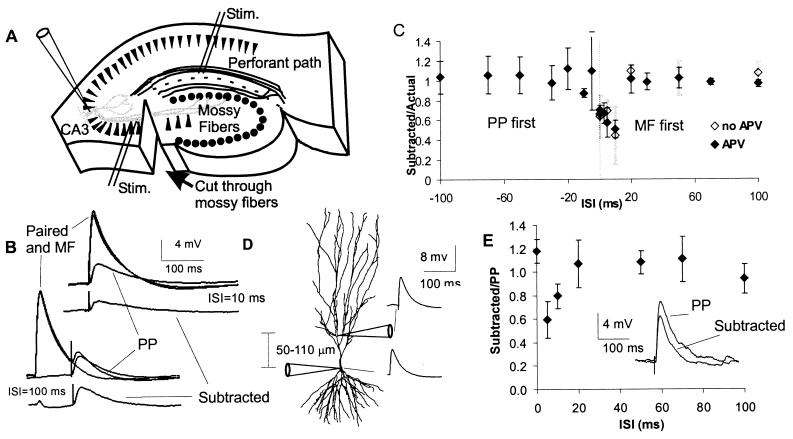
Figure 1.
Perforant path and mossy fiber EPSPs sum sublinearly. (A) Schematic of hippocampal slice showing positions of stimulating and recording electrodes and the location of a cut made through the slice to prevent disynaptic activation via mossy fiber synapses. (B) Perforant path EPSPs are suppressed by mossy fiber stimulation at 10 ms but not at 100 ms prior to perforant path stimulation. Perforant path and mossy fiber-evoked EPSPs are shown along with EPSPs resulting from the paired stimulation (Paired) at 10-and 100-ms ISIs. Also shown is the waveform resulting from the subtraction of the mossy fiber EPSP from the paired EPSP (Subtracted). (C) Ratio of amplitude of subtracted EPSP to the amplitude of the actual second EPSP is plotted for various ISIs from −100 to +100 ms. Negative ISIs indicate that perforant path stimulation came before mossy fiber stimulation (N = 9 for ISIs >0, N = 6 for ISIs <0, N = 15 for ISI = 0). (D) Diagram showing the approximate positions of patch electrodes and the shape of simulated mossy fiber EPSPs for double patch recordings. (E) Linearity of summation versus ISI shown for experiments in which mossy fiber synaptic activation was replaced by an α function injected into the proximal apical dendrite of the CA3 cell, 50–100 μm from the somatic recording site (N = 4).
METHODS
Electrophysiological Methods.
Rat hippocampal slices (400 μm) were prepared according to procedures described previously (17). Slices were cut through the mossy fiber pathway (Fig. 1A) in CA3 with a scalpel immediately after being sectioned on the vibratome. For most experiments, slices were obtained from rats 24 to 40 days old. For experiments involving double patch clamp recording from soma and apical dendrites, the animals used were 18–21 days old. Subsequently, summation of EPSPs was characterized in slices from 17- to 21-day-old animals using standard synaptic stimulation and found to be not significantly different from the data reported in Fig. 1C. Slices were incubated at 37°C for 1 hr after slicing in an artificial cerebrospinal fluid containing NaCl, 125 mM; KCl, 2 mM; dextrose, 10 mM; NaHCO3, 26 mM; MgCl, 6 mM; CaCl2, 1 mM, and then returned to room temperature. Whole-cell recordings were obtained using Axoclamp 2A and Axopatch 1D amplifiers and custom software written in LabView (National Instruments, Austin, TX). Cells were visualized using infrared differential interference contrast optics (Zeiss Axioskop) and video microscopy (Hamamatsu, Middlesex, NJ). Whole-cell pipettes (3–7 MΩ, 7–12 MΩ for dendritic recordings) were pulled from borosilicate glass and filled with solutions containing either potassium gluconate, 120 mM; KCl, 20 mM; Hepes, 10 mM; EGTA, 1 mM; Mg-ATP, 4 mM; Na-GTP, 0.3 mM; and sodium phosphocreatine, 10 mM, or in the four cells described in Fig. 2B: CsF, 120 mM; CsCl, 20 mM; EGTA, 10 mM; Hepes, 10 mM; and QX-314, 5 mM. Recordings were made in the artificial cerebrospinal fluid described above, except that the CaCl2 and MgCl2 concentrations were changed to 3 mM, and bicuculline (10–20 μM, Sigma), CGP35348 (500 μM), and D-APV (25 μM, Tocris Neuramin, Bristol, U.K.) were added to isolate the AMPA receptor-dependent EPSPs. CGP35348 was a gift from CIBA–Geigy and the QX-314 was a gift from Alamone Labs (Jerusalem, Israel). Stimulation electrodes were positioned in the Stratum lacunosum moleculare and Stratum lucidum to activate perforant path and mossy fibers, respectively. Previous work (12, 18) demonstrated that these stimulation locations resulted in activation of the desired synaptic inputs.
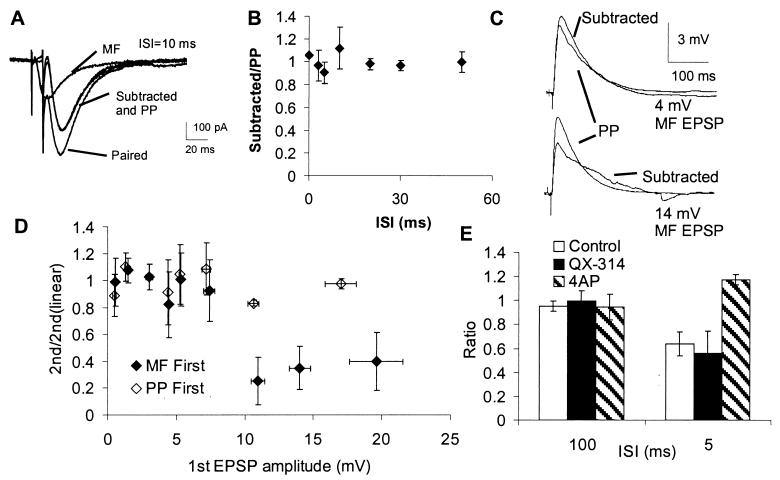
Figure 2.
Sublinear summation is voltage dependent and mediated by transient potassium channels. (A) Using a protocol similar to that of Fig. 1B, but performing the experiments in voltage clamp, perforant path and mossy fiber EPSCs sum linearly at a 10-ms ISI. Electrode contained K+ gluconate-based pipette solution. (B) Linear summation was observed at all ISIs tested. Cells held in voltage clamp at −75 mV. Whole-cell electrodes containing either K+ gluconate (N = 5) or cesium fluoride and QX-314 (N = 4) produced similar results. (C) Summation is linear when the amplitude of the preceding mossy fiber EPSP is 4 mV, but sublinear when it is 14 mV (ISI = 5 ms, Vm = −70 mV). (D) Data showing the relationship between mossy fiber (N = 7) and perforant path (N = 5) EPSP amplitude and the ratio of the amplitude of subtracted to predicted responses. (E) Summation at both the 100-ms and 5-ms ISIs is linear when the CA3 cell is filled with 4AP (5 mM), a blocker of voltage-dependent potassium channels, but remains sublinear when pipette solution contains QX-314 (10 mM).
Simulations.
Compartmental models of reconstructed CA3 pyramidal cells (19) were implemented in GENESIS. Passive parameters were: Rm = 30,000 Ωcm2 (soma) or 20,000 Ωcm2 (dendrites), Ri = 150 Ωcm, Cm = 1.0(soma) or 1.5 μF/cm2 (dendrites). The differences in passive parameters between soma and dendrites was used to account for membrane in spines, which were not modeled explicitly. Synaptic conductance changes were simulated using the standard GENESIS synchan object with time constants of 0.5 and 5 ms. Synaptic inputs were simulated by placing 2–10 individual synapses in apical dendritic segments either >450 μm (perforant path) or <150 μm (mossy fiber) from the soma.
RESULTS
We examined the summation of perforant path and mossy fiber synaptic inputs to CA3 pyramidal neurons to determine whether voltage-dependent channels influence the way in which these cells integrate synaptic inputs. The propagation of perforant path EPSPs to the CA3 cell soma is amplified by subthreshold activation of voltage-dependent conductances (20) in a manner similar to that observed in other pyramidal cells (21–23). We therefore hypothesized that somatic EPSPs evoked by perforant path stimulation would be modulated by changes in membrane potential and that they would sum actively and nonlinearly with other voltage changes (including EPSPs).
To study the linearity of summation of EPSPs, we evoked mossy fiber and perforant path EPSPs singly and also in pairs with interstimulus intervals (ISIs) of 0–100 ms while recording from CA3 pyramidal cells. In some experiments mossy fiber stimulation preceded perforant path stimulation (ISI > 0 ms) and in others, the order was reversed (ISI < 0 ms), allowing us to assess the effect of prestimulation of each of these pathways on the other.
For positive ISIs (mossy fiber first), the response recorded following the stimulation of the perforant path alone was compared with the contribution of the perforant path to the combined response, as determined by subtracting the mossy fiber response from the response resulting from paired stimulation (Fig. 1B). This subtraction of waveforms resulted in a fourth waveform which we termed the “subtracted” response. The ratio of the peak amplitude of this subtracted response to that of the actual perforant path response was used as a measure of the nonlinearity of the summation of EPSPs. A ratio of 1 indicates linear summation; ratios of greater and less than 1 indicate supralinear and sublinear summation, respectively. The analogous procedure (subtracting perforant path EPSPs from the paired EPSP and comparing the result to the mossy fiber EPSP) was used for experiments in which perforant path synapses were stimulated first (negative ISIs).
Prestimulation of mossy fibers resulted in sublinear summation of EPSPs for ISIs between 0 and 10 ms, with the maximal nonlinearity being a 49 ± 9% reduction of the perforant path EPSP for a 10-ms ISI (Fig. 1C). At intervals between 20 and 100 ms and at all negative ISIs (when the perforant path was stimulated before mossy fibers), the distal and proximal inputs summed linearly (Fig. 1A). The reasons for the asymmetry (mossy fiber prestimulation affecting perforant path EPSPs but not the reverse) are explored below. Note that, unlike the sublinearity predicted by passive models (1) (see Fig. 4B), the duration of the sublinearity (≈15 ms) did not reflect the time course of the mossy fiber EPSP (which decays more slowly) excitatory postsynaptic current or the AMPA receptor-mediated excitatory postsynaptic current (which is faster). Summation of EPSPs was unaffected by blockade of NMDA receptors (Fig. 1C).
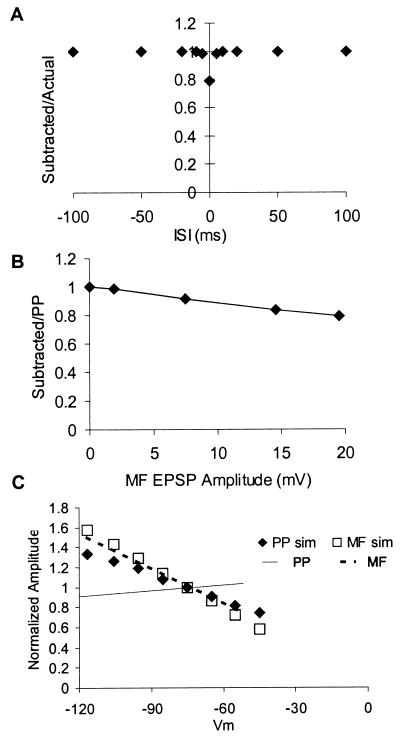
Figure 4.
Simulations show that passive model cannot account for observed nonlinearities. Unless otherwise stated, data from simulations were analyzed in the same manner as data from the experiments. (A) Compare with Fig. 2D. Simulations show the effect of mossy fiber EPSP amplitude on the linearity of EPSP summation. Passive sublinearity increases almost linearly with mossy fiber EPSP amplitude, with a 20-mV EPSP resulting in the subtracted response being 80% of the unpaired response. (B) Compare with Fig. 1C. The dependence of summation on ISI for this same simulated 20-mV EPSP shows that the passive sublinearity is restricted to very short ISIs. (C) Compare with Fig. 3A. (B) Comparison of simulated and actual data on the effect of somatic depolarization on perforant path and mossy fiber EPSPs. The voltage dependence of perforant path EPSPs (——) line, expressed as the average best fit line through data from like those shown in Fig. 3A taken from N = 12 cells) is inconsistent with the voltage dependence predicted for synapses on the distal-most dendrites of a CA3 cell (♦). The voltage dependence of real (–––, N-6) and simulated (□) mossy fiber EPSPs are almost identical.
Sublinear summation could result from the passive mechanisms (changes in reversal potential or input resistance) described above (see Introduction). Alternatively, sublinearity could result from activation of a voltage-dependent channel, resulting in outward current flow and reduced depolarization or inactivation of voltage-dependent sodium or calcium channels involved in boosting of the perforant path EPSP. A sublinearity resulting from either of the first two mechanisms would be predicted by passive cable theory and thus we term these to be examples of “passive nonlinear summation,” whereas the third and fourth mechanisms we term examples of “active nonlinear summation.” Passive nonlinearities summation has been observed previously in motoneurons (24), but, notably, not in hippocampal neurons (25, 26).
To test whether the observed sublinear summation was a result of the shunting of perforant path synaptic current by the mossy fiber synaptic conductance, we replaced mossy fiber stimulation with direct current injection into the apical dendrite of the pyramidal cell via a patch pipette. The current injected into the dendrite was in the form of an α function, the parameters of which were selected to approximate mossy fiber EPSPs (Fig. 1D). In these experiments, perforant path EPSPs were reduced in a manner similar to that observed in experiments in which mossy fibers were stimulated. The maximal reduction was 40 ± 16% at an ISI of 10 ms (Fig. 1E). However, unlike the case of synaptic stimulation, summation was linear for ISIs of 0 ms. This difference may indicate that the reduction of the perforant path EPSP at the 0-ms ISI is due to shunting by the mossy fiber synaptic conductance, or it may simply reflect the fact that the direct current injection produces a depolarization without the delay that results when mossy fiber axons are simulated. In these experiments the average delay between stimulation and onset of mossy fiber EPSP was 2.8 ± 0.5 ms. Thus, the 0-ms ISI in these dendritic current injection experiments is comparable to the an ISI of −2.8 ms in experiments using real mossy fiber synaptic input, at which linear summation is likely to be observed.
We next performed the same experiments while voltage clamping at −75 mV. In these recordings, distal and proximal excitatory postsynaptic currents summed linearly at all ISIs from 0 to 100 ms (Fig. 2 A and B). These results indicate that, even at the 0-ms ISI, the observed sublinear summation was mediated postsynaptically and voltage dependent, but was not caused by shunting of perforant path synaptic current by the mossy fiber synaptic conductance change. We concluded that the sublinearity of summation of perforant path and mossy fiber EPSPs was mediated actively, i.e., via opening of voltage-dependent channels in the postsynaptic cell. We were able to block sublinear summation by somatic voltage clamp, despite the fact that our voltage control of distal dendrites was probably poor due to inadequate space clamp. This suggests that the channels mediating the sublinearity are close to the soma.
We examined further the voltage dependence of this sublinear summation by examining the dependence of the linearity of summation on the peak depolarization observed during the mossy fiber EPSP. In these experiments we gave unpaired and paired (at ISIs of 5 and 100 ms) mossy fiber and perforant path stimulation, while varying the amplitude of the mossy fiber stimulation. The magnitude of the reduction of the perforant path EPSP depended, in a nonlinear fashion, on the amplitude of the mossy fiber-evoked EPSP. EPSPs of <5 mV peak amplitude resulted in no significant interaction, while EPSPs of ≈12 mV resulted in maximal interaction (Fig. 2C). Simulations (see Fig. 4A) showed that for passive nonlinearities, the magnitude of the sublinearity at the 0-ms ISI varied approximately linearly with EPSP amplitude, with a 20-mV mossy fiber EPSP resulting in a subtracted response that was 82% of the unpaired perforant path EPSP. Similar results were obtained from two cells in which the mossy fiber stimulation was replaced by direct current injection via a dendritic patch electrode.
In other experiments, the magnitude of perforant path EPSPs was varied to determine whether prestimulation of perforant path EPSPs could be shown to have some effect on mossy fiber EPSP amplitudes. Varying the amplitude of perforant path EPSPs had no significant effect on summation with mossy fiber EPSPs (Fig. 2D), suggesting either that perforant path EPSPs resulted in the opening of different types of voltege-dependent channels than did mossy fiber EPSPs. Alternatively, perforant path EPSPs may be more sensitive to prestimulation because they are significantly boosted by voltage-dependent sodium and calcium channels (20), or simply because they travel through more of the dendrite on the way to the soma (see Discussion).
Based on the voltage dependence for the sublinear summation, we hypothesized that this sublinear summation was caused either by the inactivation of the sodium or calcium channels involved in boosting the perforant path EPSP (20) or by the activation of transient A-type potassium channels (9). Blocking voltage-dependent sodium and calcium channels by the addition of 10 mM QX-314 (27) to the pipette solution had no effect on summation (Fig. 2E), suggesting that inactivation of boosting channels was not the mechanism of the sublinearity. However, in cells recorded using pipette solutions containing 4-aminopyridine (4AP; 5 mM), which blocks transient potassium channels when applied intracellularly (9, 28, 29), perforant path and mossy fiber EPSPs summed linearly at the 5- and 100-ms ISIs (the only ISIs tested; Fig. 2E), suggesting that a voltage-dependent potassium channel, most likely an A channel, is responsible for the sublinear summation observed.
We next examined the voltage dependence of mossy fiber and perforant path EPSPs as well as the summation of EPSPs. We considered experiments on voltage dependence to be simple versions of summation experiments, in which one of the voltage changes being summed is a voltage step. Thus, these experiments explored the summation of a steady-state voltage change with one or two synaptically activated voltage changes. Steady-state depolarization should inactivate A channels, and thus we expected that summation of perforant path and mossy fiber EPSPs should be more linear when combined with a steady-state depolarization of sufficient amplitude. Also, previous work has shown that synapses onto layer 3 and 5 pyramidal cells in neocortex (21, 30–33) or collateral synapses onto CA3 pyramidal cells show little change, or even an increase in amplitude with steady-state depolarization, suggesting that the amplitude of these EPSPs is affected by activation of postsynaptic voltage-dependent conductances.
On average, the amplitude of perforant path EPSPs increased (by 1.3 ± 1.3% per 10 mV, n = 12) with depolarization from resting potential over the range from −100 to −60 mV (Fig. 3 A and C). Thus, the summation of perforant path EPSPs with steady-state voltage changes was essentially linear. This insensitivity of perforant path EPSPs’ amplitude to changes in somatic membrane potential is due in part to the attenuation of the steady-state somatic voltage change with distance. However, as shown in Fig. 4C, the very small voltage dependence of the perforant path EPSP peak is inconsistent with a synaptic location even on the most distal dendrite of a CA3 cell, strongly suggesting that voltage-dependent channels are involved in the summation of perforant path EPSPs with steady-state voltage changes (Fig. 4C). Previous work (34) has suggested that this sort of anomalous voltage dependence of EPSPs may be due to activation of NMDA receptors, but these were blocked in our study. Thus, other mechanisms, such as activation of persistent sodium (21), or low voltage-activated calcium channels (33), or inactivation of the hyperpolarization-activated channels mediating “sag” (31, 35) may account for this phenomenon.
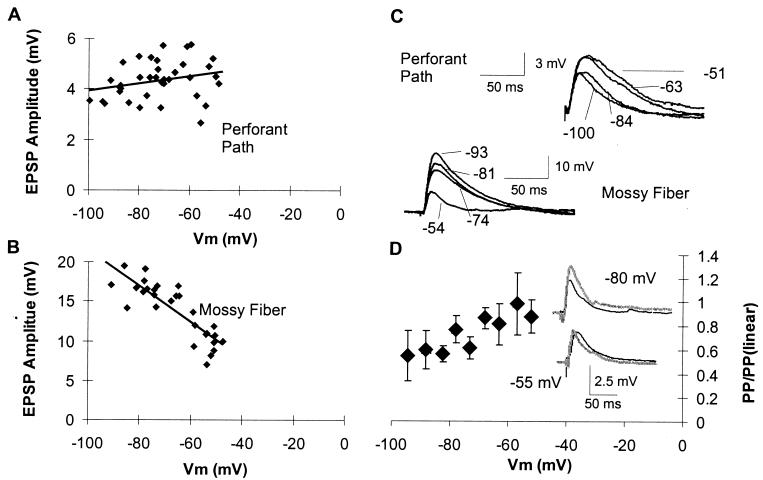
Figure 3.
Summation of EPSPs with steady-state voltage changes varies with synapse location. (A and B) Steady-state changes in membrane potential were produced by somatic current injection. Perforant path and mossy fiber EPSPs were elicited at a variety of membrane potentials. Lines represent best linear fit through these data points. The amplitude of perforant path EPSPs (A and C) did not decrease with somatic depolarization, indicating linear summation of perforant path EPSPs with steady-state changes in Vm. (B and C) In contrast, mossy fiber EPSP amplitude was reduced by depolarization consistent with the reversal potential of AMPA receptor-mediated EPSPs. (D) The linearity of summation also showed voltage dependence, with summation becoming linear with depolarization.
In contrast, mossy fiber EPSPs decreased with depolarization (average change in peak 12.7 ± 4.1% per 10 mV from −70, n = 6; Fig. 3) and had a projected reversal potential of near 0 mV, consistent with their proximal location (Fig. 4C). Thus, mossy fiber EPSPs summed sublinearly with steady-state somatic voltage changes in a manner consistent with cable theory and with the expected effect of changes in reversal potential.
Summation of perforant path and mossy fiber EPSPs also showed significant voltage dependence, with summation at 0-ms ISIs being linear for potentials more positive than −60 (0.99 ± 0.25 at −57 mV) and becoming more sublinear when membrane potential was hyperpolarized from rest. This supports our conclusion that the sublinearity was caused by the activation of a transient potassium channel, which was inactivated by the steady-state depolarization.
DISCUSSION
These results demonstrate that voltage-dependent channels play an important role in the summation of voltage changes in pyramidal cells and thus in synaptic integration. The way in which voltage changes are summed depends on the amplitude, timing, and site of initiation of the voltage changes. Summation of perforant path EPSPs with large, but not small transient depolarizations (such as mossy fiber EPSPs) was sublinear due to activation of transient potassium channels, whereas summation with steady-state voltage changes was actively linear. Summation of mossy fiber EPSPs with both transient and steady-state voltage changes was well described by passive simulations. This difference between these two inputs may in part reflect the differences in distributions of voltage-dependent channels in the proximal and distal dendritic membrane, and suggests that the relative strengths of proximal and distal inputs may change dynamically with ongoing activity.
We believe that the sublinear summation we have observed results from the activation of transient potassium channels by the mossy fiber EPSP. Recent data (36) suggest that the somatic and dendritic transient potassium current in CA1 pyramidal cells is mediated by Kv4.2 rather than any of the Kv1 (shaker) channels. Although no detailed studies of transient potassium channel types have been performed in CA3 pyramidal cells, overall transient potassium currents in CA1 and CA3 cells are quite similar (37) and anatomical data (38, 39) suggest that Kv4.2 channels are the major type of transient potassium channel in CA3 pyramidal cell soma and dendrites. The density of Kv4.2 channels in CA3 may be lower than in CA1, but Kv4.3 channels seem to be present at higher densities in CA3 than in CA1 pyramidal cells (38). Transfected Kv4.2 and Kv4.3 channels have similar inactivation kinetics (τ < 5 ms at −30 mV) and sensitivity to 4AP (EC50 ≈ 5 mM), although Kv4.3 channels activate and inactivate at more negative potentials than do Kv4.2 channels (40–42). These properties of Kv4.2 and Kv4.3 potassium channels correspond closely to the time course, sensitivity to 4AP, and inactivation with depolarization of the summation as described above. Other transient potassium channels, such as those of the Kv1 family, are sensitive to much lower concentrations of 4AP, are activated at more depolarized potentials (29), and are localized presynaptically in hippocampus (39). Thus, we believe Kv4.2 and perhaps also Kv4.3 channels to be responsible for the sublinear summation, opening the possibility that modulation of these channels may alter the properties of summation in CA3 cells (36).
The interaction between mossy fiber and perforant path EPSPs is asymmetrical in time, in that prestimulation of mossy fibers reduces perforant path EPSPs but not vice versa. This asymmetry suggests that the temporal order of firing in EC and dentate gyrus may be important for determining the level of activation in CA3 pyramidal cells. The mechanism of this asymmetry is not clear, but we believe that this effect is most likely due to the location of open A channels relative to the site of the synaptic activation and/or to the difference in time course between mossy fiber and perforant path EPSPs. Opening of A channels results in hyperpolarization and also in shunting of any synaptic current traveling through the dendritic region where the A channels are located. It is well known that the effects of shunting inhibition are larger if the shunt is opened in the path between the site of synaptic activation and the soma (2), as is likely to be the case when mossy fibers are stimulated first. When perforant path EPSP are elicited before mossy fiber EPSPs, current from mossy fibers will not pass through the regions of dendrite where A channel activation is highest, and thus they will be less affected by the preceding EPSP. Also, simulations of A channels suggest that these channels may inactivate during the slow rising phase of perforant path EPSPs (unpublished observations). Thus, EPSPs with faster rise times, such as mossy fiber EPSPs, may be more effective in activating A channels than slow EPSPs, including perforant path EPSPs.
Thus, how a neuron sums its inputs becomes a rather complex issue. Small (<5 mV) EPSPs are added linearly in all cases that we have studied, but when the summation involves larger inputs, the result depends critically on the amplitude and timing of the EPSPs being summed. Therefore, in this example of neuronal arithmetic at perforant path and mossy fiber synapses, 1 mV + 1 mV = 2 mV, but 8 mV + 8 mV <16 mV, but only if the two inputs arrive at approximately the same time. The amplitude dependence of this sublinearity suggests that such nonstandard arithmetic may be especially important when neurons receive a large input either via a specialized synapse such as the mossy fiber synapse,or via synchronization of inputs as occurs during various population oscillations.
In contrast to the predictions of modeling studies (3, 8, 10), we found no evidence for supralinear summation. Instead, we observed that perforant path EPSPs summed sublinearly with proximal EPSPs, but linearly with steady-state somatic depolarization. However, in the experiments here, the two inputs are far apart on the dendritic tree. The computational studies of synaptic integration described above focused on synapses located close to each other on the dendritic tree, and the nonlinear interactions between such synapses may differ from the synapses we describe here. Nonetheless, our results indicate that voltage-dependent sublinear summation occurs in dendrites and thus suggests that dendrites support a computational complexity not previously appreciated.
These results, as well as recent anatomical data showing that interneurons are the primary synaptic targets of mossy fibers (43), indicate that granule cell activation will have a mixed effect on CA3 pyramidal cell activity and suggest that activation of CA3 cells by mossy fiber input is more selective than previously appreciated. In sharp contrast with the view of the mossy fiber as an input that functions as “detonator synapse” (16), mossy fiber activation may function as a way of dynamically regulating the sensitivity of CA3 pyramidal cells to their array of inputs, so that the strength of a given input, or the likelihood of an input being potentiated, will depend on its arrival time relative to the most recent mossy fiber input. These data also suggest that transient or persistent modulation of potassium channels (36) may be an important mechanism for modulating distal inputs as well as their sensitivity to the arrival of other inputs.
ABBREVIATIONS
- EPSP
excitatory postsynaptic potential
- EC
entorhinal cortex
- ISI
interstimulus interval
- 4AP
4-aminopyridine
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Redman S J. Int Rev Physiol Neurophysiol. 1976;10:1–35. [Google Scholar]
- 2.Koch C, Poggio T, Torre V. Proc Natl Acad Sci USA. 1983;80:2799–2802. doi: 10.1073/pnas.80.9.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mel B W. J Neurophysiol. 1993;70:1086–1101. doi: 10.1152/jn.1993.70.3.1086. [DOI] [PubMed] [Google Scholar]
- 4.Konig P, Engel A K, Singer W. Trends Neurosci. 1996;19:130–137. doi: 10.1016/s0166-2236(96)80019-1. [DOI] [PubMed] [Google Scholar]
- 5.Rieke F, Warland D, de Ruyter van Steveninck R, Bialek W. Spikes: Exploring the Neural Code. Cambridge, MA: MIT Press; 1996. [Google Scholar]
- 6.Yuste R, Tank D W. Neuron. 1996;16:701–716. doi: 10.1016/s0896-6273(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 7.Magee J C, Hoffman D A, Colbert C M, Johnston D. Annu Rev Physiol. 1998;60:327–346. doi: 10.1146/annurev.physiol.60.1.327. [DOI] [PubMed] [Google Scholar]
- 8.Softky W. Neuroscience. 1994;58:13–41. doi: 10.1016/0306-4522(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman D A, Magee J C, Colbert C M, Johnston D. Nature (London) 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- 10.Wilson C J. J Comput Neurosci. 1995;2:91–115. doi: 10.1007/BF00961882. [DOI] [PubMed] [Google Scholar]
- 11.Steward O. J Comp Neurol. 1976;167:285–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- 12.Berzhanskaya J, Urban N N, Barrionuevo G. J Neurophysiol. 1998;79:2111–2118. doi: 10.1152/jn.1998.79.4.2111. [DOI] [PubMed] [Google Scholar]
- 13.Yeckel M F, Berger T W. Proc Natl Acad Sci USA. 1990;87:5832–5836. doi: 10.1073/pnas.87.15.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treves A, Rolls E T. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- 15.O’Reilly R C, McClelland J L. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- 16.McNaughton B L, Morris R G. Trends Neuroci. 1987;10:408–415. [Google Scholar]
- 17.Urban N N, Barrionuevo G. J Neurosci. 1996;16:4293–4299. doi: 10.1523/JNEUROSCI.16-13-04293.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapur A, Yeckel M F, Johnston D. J Neurophysiol. 1998;79:2181–2190. doi: 10.1152/jn.1998.79.4.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henze D A, Cameron W E, Barrionuevo G. J Comp Neurol. 1996;369:331–344. doi: 10.1002/(SICI)1096-9861(19960603)369:3<331::AID-CNE1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Urban, N. N., Henze, D. A. & Barrionuevo, G. (1998) J. Neurophysiol., in press. [DOI] [PubMed]
- 21.Stuart G, Sakmann B. Neuron. 1995;15:1065–1076. doi: 10.1016/0896-6273(95)90095-0. [DOI] [PubMed] [Google Scholar]
- 22.Lipowsky R, Gillessen T, Alzheimer C. J Neurophysiol. 1996;76:2181–2191. doi: 10.1152/jn.1996.76.4.2181. [DOI] [PubMed] [Google Scholar]
- 23.Gillessen T, Alzheimer C. J Neurophysiol. 1997;77:1639–1643. doi: 10.1152/jn.1997.77.3.1639. [DOI] [PubMed] [Google Scholar]
- 24.Burke R E. J Neurophysiol. 1967;30:1114–1137. doi: 10.1152/jn.1967.30.5.1114. [DOI] [PubMed] [Google Scholar]
- 25.Langmoen I A, Andersen P. J Neurophysiol. 1983;50:1320–1329. doi: 10.1152/jn.1983.50.6.1320. [DOI] [PubMed] [Google Scholar]
- 26.Cash S, Yuste R. J Neurosci. 1998;18:10–15. doi: 10.1523/JNEUROSCI.18-01-00010.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talbot M J, Sayer R J. J Neurophysiol. 1996;76:2120–2124. doi: 10.1152/jn.1996.76.3.2120. [DOI] [PubMed] [Google Scholar]
- 28.Stephens G J, Garratt J C, Robertson B, Owen D G. J Physiol. 1994;477:187–196. doi: 10.1113/jphysiol.1994.sp020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bossu J L, Capogna M, Debanne D, Mckinney R A, Gahwiler BH. J Physiol. 1996;495:367–381. doi: 10.1113/jphysiol.1996.sp021600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cauller L J, Connors B W. J Neurosci. 1994;14:751–762. doi: 10.1523/JNEUROSCI.14-02-00751.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicoll A, Larkman A, Blakemore C. J Physiol. 1993;468:693–710. doi: 10.1113/jphysiol.1993.sp019795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomson A M, Deuchars J, West D C. J Neurophysiol. 1993;70:2354–2369. doi: 10.1152/jn.1993.70.6.2354. [DOI] [PubMed] [Google Scholar]
- 33.Deisz R A, Fortin G, Zieglgansberger W. J Neurophysiol. 1991;65:371–382. doi: 10.1152/jn.1991.65.2.371. [DOI] [PubMed] [Google Scholar]
- 34.Thomson A M, Girdlestone D, West D C. J Neurophysiol. 1988;60:1896–1907. doi: 10.1152/jn.1988.60.6.1896. [DOI] [PubMed] [Google Scholar]
- 35.Spruston N, Jaffe D B, Williams S H, Johnston D. J Neurophysiol. 1993;70:781–802. doi: 10.1152/jn.1993.70.2.781. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman D A, Johnston D. J Neurosci. 1998;18:3521–3528. doi: 10.1523/JNEUROSCI.18-10-03521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klee R, Ficker E, Heinemann U. J Neurophysiol. 1995;74:1982–1995. doi: 10.1152/jn.1995.74.5.1982. [DOI] [PubMed] [Google Scholar]
- 38.Serodio P, Rudy B. J Neurophysiol. 1998;79:1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- 39.Sheng M, Tsaur M L, Jan Y N, Jan L Y. Neuron. 1992;9:271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- 40.Pak M D, Baker K, Covarrubias M, Butler A, Ratcliffe A, Salkoff L. Proc Natl Acad Sci USA. 1991;88:4386–4390. doi: 10.1073/pnas.88.10.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serodio P, Kentros C, Rudy B. J Neurophysiol. 1994;72:1516–1529. doi: 10.1152/jn.1994.72.4.1516. [DOI] [PubMed] [Google Scholar]
- 42.Serodio P, Vega-Saenz d M, Rudy B. J Neurophysiol. 1996;75:2174–2179. doi: 10.1152/jn.1996.75.5.2174. [DOI] [PubMed] [Google Scholar]
- 43.Acsady L, Kamondi A, Sik A, Freund T, Buzaki G. J Neurosci. 1998;18:3386–403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]