Abstract
Serotonin (5-hydroxytryptamine) type 3 receptors (5-HT3R) and nicotinic acetylcholine receptors are structurally and functionally related proteins, yet distinct members of the family of ligand-gated ion channels. For most members of this family a diversity of heteromeric receptors is known at present. In contrast, known 5-HT3R subunits are all homologs of the same 5-HT3R-A subunit and form homopentameric receptors. Here we show, by heterologous expression followed by immunoprecipitation, that 5-HT3R and nicotinic acetylcholine receptor α4 subunits coassemble into a novel type of heteromeric ligand-gated ion channel, which is activated by 5-HT. The Ca2+ permeability of this heteromeric ion channel is enhanced as compared with that of the homomeric 5-HT3R channel. Heteromeric 5-HT3/α4 and homomeric 5-HT3Rs have similar pharmacological profiles, but distinct sensitivities to block by the antagonist d-tubocurarine. Coassembly of subunits beyond the boundaries of ligand-gated ion channel families may constitute an important mechanism contributing to the diverse properties and functions of native neurotransmitter receptors.
Through molecular cloning a plethora of subunits of ligand-gated ion channels has been identified. In heterologous expression systems, the coassembly of related subunits permits formation of a vast repertoire of neurotransmitter receptors, each with its own characteristic properties. A focus in contemporary neuroscience is to understand the functional significance of the variety of cloned subunits for the diverse properties and functions of native neurotransmitter receptors. For serotonin (5-hydroxytryptamine) type 3 receptors (5-HT3R), little molecular diversity has become apparent: only a single class of 5-HT3R subunit has been cloned (1–3), and these subunits form homomeric receptors with similar functional properties in heterologous expression systems (4, 5). Conversely, studies of native receptors, indicating substantial heterogeneity of 5-HT3R properties, suggest the existence of additional subunits involved in the formation of heteromeric 5-HT3Rs (6–9).
Ligand-gated ion channels share homology in their primary and predicted secondary structures. A particularly close relationship between 5-HT3R and nicotinic acetylcholine receptors (nAChR) has been demonstrated by the construction of a functional chimeric receptor, containing the ligand-binding domain of the nAChR α7 and the ion channel domain of the 5-HT3R subunits (10). Because of the close resemblance between 5-HT3 and nAChR subunits, we examined whether the 5-HT3R subunit can coassemble with nAChR subunits to form heteromeric receptors.
MATERIALS AND METHODS
Expression and Recording from Xenopus Oocytes.
Oocytes from mature specimens of Xenopus laevis were harvested, injected, and incubated as described before (11). cDNA encoding the 5-HT3R-A and cDNAs encoding the α2, α3, α4, α7, β2, and β4 nAChR subunits were injected into the nucleus either alone or pairwise at a ratio of 1:3 5-HT3R-A/nAChR cDNA (total injection volume ≈32 nl). For experiments under Cl−-free conditions, oocytes were incubated in Cl−-free modified Barth’s solution as described before (11). Ion currents were recorded from oocytes 2–5 days after injection by conventional two-microelectrode voltage clamp. The membrane potential was held at −60 mV or at −20 mV, unless otherwise noted. Microelectrodes (≤1 MΩ) were filled with 3 M KCl, or with 3 M K-methanesulfonate and 50 mM KCl for experiments performed under Cl−-free conditions. Oocytes were continuously superfused with external solution containing 115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 10 mM Hepes, pH 7.2 with NaOH). For recordings under Cl−-free conditions the same external solution was used with methanesulfonate substituting for Cl−. For recordings under Ca2+-free conditions external solution with 1 mM Mg2+ substituting for Ca2+ was used. For chelation of intracellular Ca2+, 50 nl of a 50 mM BAPTA [bis(2-aminophenoxy)ethane-N, N,N′,N′-tetraacetic acid] solution was injected into oocytes during the experiments via a third micropipette by using a Drummond microinjector. All agonists used were applied at near maximum-effective concentrations (5, 12).
Expression and Immunoprecipitation from Human Embryonic Kidney (HEK) 293 Cells.
HEK 293 cells were transfected by calcium phosphate precipitation (13) by using the eukaryotic expression vector pRc/CMV containing cDNA for the 5-HT3R, the nAChR α4, the nAChR β2, or the myc-tagged γ-aminobutyric acid type A receptor (GABAAR) α1 subunit. After transfection cells were incubated for 72 hr at 3% CO2. Preparation of membranes from transiently transfected cells, solubilization of membranes, and concentration of membranes for direct immunoblots were performed according to previously described methods (14). Receptors were immunoprecipitated by using 5 μg of subunit-specific antibody, followed by collection using a mixture of protein A and G-Sepharose beads. After three washes in lysis buffer, the beads were resuspended in sample buffer, and samples subjected to SDS/PAGE on a 12% polyacrylamide gel. Proteins were transferred to nitrocellulose membrane and incubated in either mAb 299 (1:1,000), pAb 5-HT3 (1:350), or bd24 (1:5) for 1 hr. After three 5-min washes in PBS containing 0.05% Tween 20, antibodies were labeled for 1 hr with anti-rat (mAb 299), anti-rabbit (pAb 5-HT3), or anti-mouse (bd24) IgG coupled to horseradish peroxidase diluted 1:1,000, and visualized by using enhanced chemiluminescence. On some of the blots a quantitative analysis of the precipitated products was performed by densitometry (Molecular Dynamics).
Calcium Imaging.
Fura-2 calcium imaging was performed on HEK 293 cells grown in the presence of 100 μM d-tubocurarine after transfection. Procedures were as described previously (15), with minor modifications to the external solution, which contained 115 mM NaCl, 5 mM KCl, 0.5 mM MgSO4, 2 mM CaCl2, 25 mM Hepes, 15 mM glucose, pH 7.4. The average resting levels of [Ca2+]i in HEK 293 cells expressing homomeric 5-HT3 or heteromeric 5-HT3/α4 receptors amounted to 53.4 ± 5.1 nM and 33.3 ± 7.9 nM, respectively (n = 6). Experimental conditions of Fura-2 calcium imaging on Xenopus oocytes were the same as described previously (16).
RESULTS
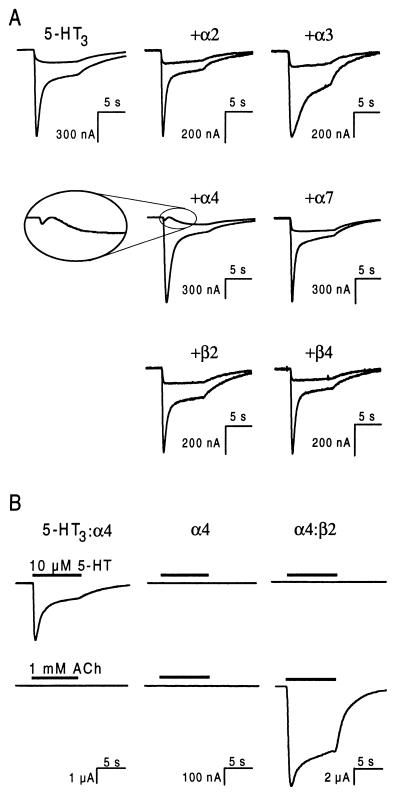
5-HT3R and nAChR subunits were coexpressed in Xenopus oocytes and the agonist-evoked ion currents were compared with those in oocytes expressing homomeric 5-HT3Rs. Inward currents induced in oocytes expressing homomeric 5-HT3Rs by superfusion with 10 μM 5-HT were similar to those described previously (1, 4, 5, 16) (Fig. 1A). Application of 5-HT to oocytes, coinjected with 5-HT3R subunit cDNA and cDNA encoding nAChR subunits α2, α3, α7, β2, or β4, yielded responses indistinguishable from those of homomeric 5-HT3Rs. However, the combination of 5-HT3R and α4 subunits yielded a biphasic response at a holding potential of −20 mV (24 of 24 oocytes, eight frogs), whereas the ion current mediated by homomeric 5-HT3Rs was always monophasic (Fig. 1A). Oocytes injected with α4 cDNA alone did not respond to 5-HT nor to acetylcholine (ACh), although the amount of α4 cDNA was sufficient for the expression of functional heteromeric nAChR when coinjected with β2 cDNA (Fig. 1B). These results show that coexpression of nAChR α4 subunits with 5-HT3R subunits alters the properties of the 5-HT3R-mediated ion current and suggest that this is caused by coassembly of 5-HT3R and α4 subunits.
Figure 1.
Coexpression of 5-HT3R and nAChR α4 subunits alters the kinetics of the 5-HT-induced ion current. (A) Ion currents evoked with 10 μM 5-HT at holding potentials of −60 mV and −20 mV (large and small responses, respectively) in oocytes expressing the 5-HT3R subunit alone (Upper Left) or a combination of the 5-HT3R subunit and the indicated nAChR subunit. (Inset) The current evoked at −20 mV in oocytes expressing 5-HT3R and nAChR α4 subunits is biphasic, whereas the corresponding currents from all other subunit combinations are monophasic and indistinguishable. (B) Ion currents evoked with either 10 μM 5-HT or 1 mM ACh in the same oocyte injected with cDNAs encoding the subunits indicated. The lower right trace shows that the amount of α4 cDNA injected was sufficient to obtain a large ACh-evoked ion current through heteromeric nAChR. The holding potential was −60 mV in all oocytes.
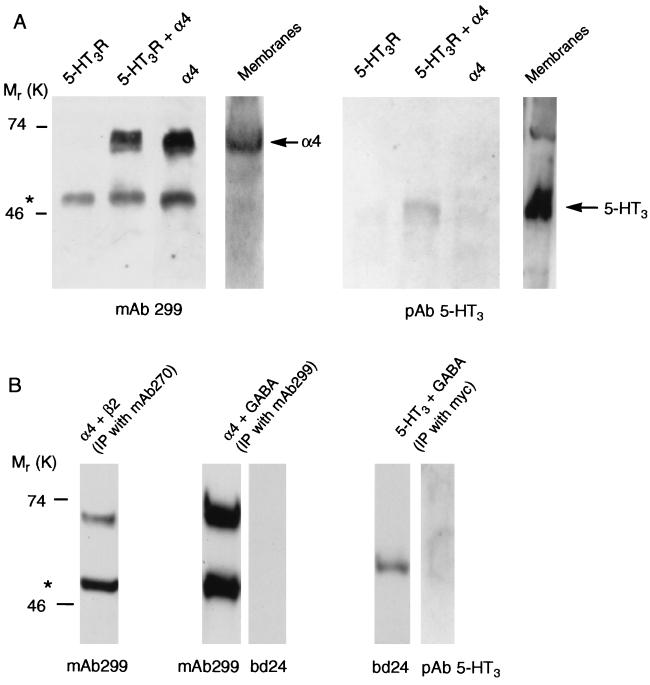
Formation of heteromeric 5-HT3/α4 receptors was demonstrated by transiently transfecting plasmids encoding these two subunits into HEK 293 cells followed by immunoprecipitation by using a mAb to the α4 subunit (mAb 299). Under the conditions used, mAb 299 will immunoprecipitate complete receptors, whose subunit size and composition then can be revealed by further analysis. By using denaturating gels followed by Western blots, labeling of the mAb 299-precipitated product by both mAb 299 and a polyclonal 5-HT3R specific antibody is demonstrated (Fig. 2A). This result shows that the original protein contained both 5-HT3R and α4 subunits. Immunoprecipitation applied to cells that were transfected with plasmids containing either the α4 subunit or the 5-HT3R subunit did not result in protein labeled by the alternative antibody (Fig. 2A). Control experiments, using the nAChR β2 subunit-specific mAb 270 (17) under identical conditions as above, show that nAChR α4 and β2 subunits coprecipitate. This finding is consistent with the notion that α4 and β2 subunits form functional heteromeric nAChR. Conversely, neither the 5-HT3R subunit nor the nAChR α4 subunit coprecipitate with the myc-tagged GABAAR α1 subunit (Fig. 2B). These results demonstrate the specific coassembly of 5-HT3R and nAChR α4 subunits into heteromeric receptors.
Figure 2.
Coprecipitation of 5-HT3R and nAChR α4 subunits. (A) Western blots of proteins precipitated by mAb 299 from HEK 293 cells transfected with the cDNAs indicated, and probed with either α4 (mAb 299) or 5-HT3 (pAb 5-HT3) specific antibodies. Direct immunoblots of membranes from cells transfected with 5-HT3R and nAChR α4 subunits also are shown. For the immunoprecipitations 1 mg of membrane protein was used and in the membranes lane 0.33 mg of protein was loaded. (B) Control experiments show that proteins precipitated from HEK 293 cells expressing nAChR α4 and β2 subunits by the β2-specific antibody mAb 270 also contain α4 subunits (left lane). Densitometric analysis showed that 4–11% of 5-HT3R (n = 3) and 31–54% of β2 subunits (n = 3) coprecipitate with α4 subunits. Conversely, myc-tagged GABAAR α1 subunits do not coprecipitate with nAChR α4 subunits (middle two lanes) and 5-HT3R subunits do not coprecipitate with myc-tagged GABAAR α1 subunits (right two lanes) as shown by the staining of duplicate samples with mAb 299 and bd24, and with bd24 and pAb 5-HT3, respectively. Antibodies used for immunoprecipitation and for staining of the precipitated proteins are indicated above and below the panels, respectively. In both A and B ∗ indicates mAb 299 heavy chain.
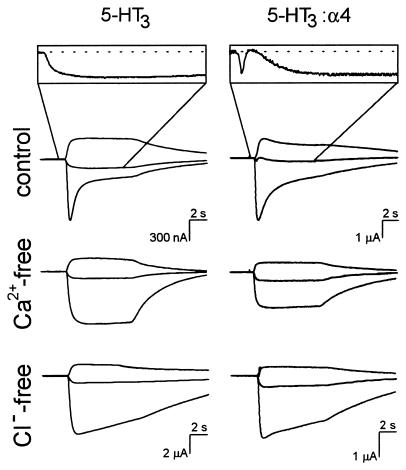
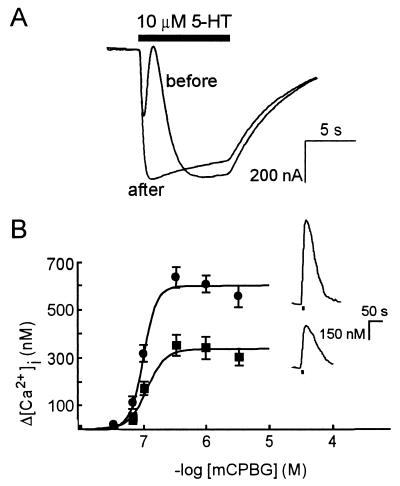
The biphasic nature of 5-HT3/α4 receptor-mediated ion current in oocytes depends on the presence of Ca2+ and Cl−. Currents evoked in oocytes expressing 5-HT3/α4 receptors in the absence of either Ca2+ or Cl− are monophasic and indistinguishable from the currents mediated by homomeric 5-HT3R (6 of 6 oocytes, three frogs; Fig. 3). This finding indicates that 5-HT3/α4 channel opening results in Ca2+ entry and secondary activation of Ca2+-dependent Cl− channels, which are natively expressed in Xenopus oocytes (18). Attempts to confirm Ca2+ entry upon activation of 5-HT3/α4 receptors in oocytes by using Fura-2 Ca2+ imaging did not result in detectable signals, whereas injection of inositol 1,4,5-trisphosphate into the same oocytes gave robust increases in intracellular [Ca2+] (not shown). This finding suggests that Ca2+ entry is local and that amounts of Ca2+ were too low to be detected, consistent with the earlier observation that activation of highly Ca2+-permeable NMDA receptors causes only modest Fura-2 signals in oocytes (16). However, the Ca2+ entry through heteromeric 5-HT3/α4 receptors in oocytes was confirmed by using the Ca2+ chelator BAPTA. The Ca2+-dependent component of the biphasic current was completely abolished after intracellular injection of BAPTA (3 of 3 oocytes, two frogs; Fig. 4A). Moreover, enhanced Ca2+ entry through heteromeric 5-HT3/α4 receptors was directly demonstrated by using Fura-2 Ca2+ imaging in HEK 293 cells by activation of the receptors with the 5-HT3R agonist meta-chlorophenylbiguanide (mCPBG). Compared with HEK 293 cells expressing homomeric 5-HT3Rs, the maximum receptor-mediated Ca2+ entry in HEK 293 cells expressing heteromeric 5-HT3/α4 receptors was ≈2-fold enhanced from 329 ± 18 nM to 594 ± 18 nM, respectively (Fig. 4B; n = 6). The values of the EC50 and Hill coefficients obtained from the concentration-effect curves of mCPBG on homomeric and heteromeric receptors were indistinguishable (Fig. 4B; 111 ± 16 nM and 3.3 ± 1 for homomeric receptors; 97 ± 5 nM and 4.1 ± 0.9 for heteromeric receptors, n = 6). The combined electrophysiological and Ca2+ imaging data demonstrate that the Ca2+ permeability of heteromeric 5-HT3/α4 receptors is significantly enhanced as compared with that of homomeric 5-HT3Rs.
Figure 3.
Enhanced Ca2+-permeability of heteromeric 5-HT3/α4 receptors expressed in oocytes. Ion currents mediated by homomeric 5-HT3Rs (Left) and heteromeric 5-HT3/α4 receptors (Right) were evoked at holding potentials of −60, −20, and +20 mV (bottom to top traces) with 10 μM 5-HT under control, Ca2+-free, and Cl−-free conditions (see Materials and Methods). Traces recorded under Ca2+-free conditions were obtained from the same oocytes used to record control traces. (Insets) Magnified current traces recorded at the holding potential of −20 mV.
Figure 4.
Heteromeric 5-HT3/α4 receptors mediate an increase in intracellular [Ca2+]. (A) Ion currents evoked with 10 μM 5-HT (filled bar) at −20 mV, before and 5 min after intracellular injection of the Ca2+ chelator BAPTA. The superimposed traces were obtained from the same oocyte. (B) Fura-2 fluorescence concentration-effect curves of mCPBG-induced increases in [Ca2+]i in HEK 293 cells expressing homomeric 5-HT3Rs (■) or heteromeric 5-HT3/α4 receptors (•). Data points are mean ± SD of six cells. Absence of error bars indicates that the SD is smaller than the symbol size. (Insets) The increase in [Ca2+]i evoked with 1 μM mCPBG in HEK 293 cells expressing homomeric 5-HT3Rs (Lower) or heteromeric 5-HT3/α4 receptors (Upper).
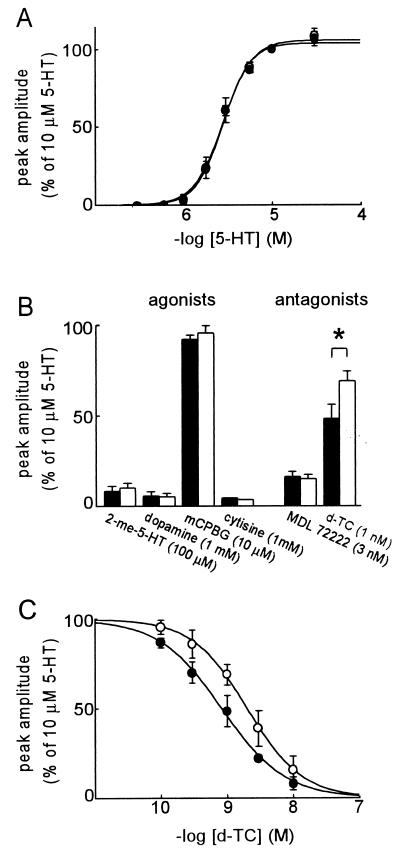
The pharmacological profiles of the 5-HT3 and 5-HT3/α4 receptors are very similar. The concentration-effect curves of 5-HT are indistinguishable (Fig. 5A), with EC50 and Hill coefficients of 2.8 ± 0.2 μM and 2.8 ± 0.3 for homomeric receptors, and 2.8 ± 0.5 μM and 2.7 ± 0.3 for heteromeric receptors (n = 3). Homomeric and heteromeric receptors are activated by the 5-HT3R agonists 2-methyl-5-HT, dopamine, and mCPBG (Fig. 5B), and not by the nAChR agonists ACh (Fig. 1B), tetramethylammonium, nicotine, and physostigmine (not shown). The nAChR agonist cytisine acts as a partial agonist on 5-HT3 and 5-HT3/α4 receptors, with an efficacy of 3–5% (Fig. 5B). None of the agonists discriminates between homomeric and heteromeric receptors. Both receptors also are inhibited by a low concentration of the selective 5-HT3R antagonist MDL 72222 (Fig. 5B). The 5-HT3R and nAChR antagonist d-tubocurarine is less potent in blocking heteromeric than homomeric receptors (Fig. 5 B and C). The IC50 of d-tubocurarine on the 5-HT3/α4 receptor (2.4 ± 0.4 nM) is significantly higher than that on the 5-HT3R (0.8 ± 0.2 nM; n = 3; Student’s t test: P = 0.02). The Hill coefficients are indistinguishable (−1.1 ± 0.1 and −0.9 ± 0.1, respectively).
Figure 5.
Pharmacological profiles of heteromeric 5-HT3/α4 receptors and homomeric 5-HT3Rs. (A) Superimposed concentration-effect curves of 5-HT on 5-HT3 (•) and 5-HT3/α4 receptors (○). (B) Agonist and antagonist sensitivities of 5-HT3 (filled bars) and 5-HT3/α4 receptors (empty bars). ∗ indicates significant difference (t test: P < 0.05). (C) Concentration-effect curves for the inhibition of the 5-HT-evoked ion current by d-tubocurarine on 5-HT3 (•) and 5-HT3/α4 receptors (○). All data points represent mean of the current amplitudes, normalized to the amplitude of the current evoked with 10 μM 5-HT, with SD bars from 3–5 oocytes (except cytisine: n = 2).
DISCUSSION
The results demonstrate that 5-HT3R and nAChR α4 subunits coassemble into a novel type of heteromeric 5-HT3 receptor channel with enhanced Ca2+ permeability and reduced sensitivity to the antagonist d-tubocurarine as compared with the homomeric 5-HT3 receptor-gated ion channel. These findings have significant implications, both for 5-HT3R pharmacology and function and for ligand-gated ion channels in general.
Since the cloning of the 5-HT3R subunit in 1991 (1), no additional class of 5-HT3R subunit has been found. There has been some interest in long and short splice variants of the 5-HT3R subunit that have been identified in rodent, but not human, tissues (2, 3, 19, 20). In heterologous expression systems both subunits form functional, homomeric ligand-gated ion channels with similar pharmacological and biophysical properties (4, 5). Despite the limited molecular diversity of the 5-HT3R, several lines of evidence suggest that at least some native 5-HT3Rs are not solely composed of either long or short 5-HT3R subunits. For example, the pharmacological profiles of 5-HT3Rs composed of either long or short subunits are different from those of 5-HT3Rs native to N1E-115 neuroblastoma cells (5, 9). In addition, 5-HT3Rs in membranes from mouse brain and ileum appear to have different pharmacological profiles (7). The present finding, that it is possible to form heteromeric 5-HT3Rs with properties distinct from those of homomeric 5-HT3Rs, provides a lead for further investigations into the subunit composition and heterogeneity of native 5-HT3Rs. As shown in Fig. 1, coexpression of nAChR α2, α3, α7, β2 ,and β4 subunits with the 5-HT3R subunit did not alter specific kinetic properties of the 5-HT-induced ion current. However, the possibility that these nAChR subunits also coassemble with the 5-HT3R subunit cannot be excluded at present.
Coassembly of 5-HT3R with α4 subunits results in ion channels with enhanced Ca2+ permeability as compared with homomeric 5-HT3Rs. Because of the importance of Ca2+ in cellular signaling, there has been considerable discussion in the literature about the level of Ca2+ entry through 5-HT3Rs. It previously has been reported that the Ca2+ permeability of 5-HT3Rs in N18 cells is relatively high (21). However, in a more recent study of recombinant 5-HT3Rs expressed in oocytes no increase in Fura-2 fluorescence was found (16). Conversely, recombinant 5-HT3Rs expressed in HEK 293 cells, and 5-HT3Rs native to N1E-115 cells, do mediate an increase in intracellular Ca2+ detectable by Fura-2 (15). As the present results confirm that Ca2+ signals induced by 5-HT3R activation in oocytes are much weaker than in HEK cells, the inconsistency in the previously published data appears mainly because of differences in receptor density and surface-to-volume ratio in oocytes, HEK 293, and neuroblastoma cells. Thus, although Ca2+ permeates through homomeric 5-HT3Rs, the level of Ca2+ entry may be too low to activate intracellular signaling pathways.
The enhanced Ca2+ permeability of heteromeric 5-HT3/α4 receptors (Figs. 3 and 4) is of particular importance in view of the alleged role of 5-HT3Rs in the presynaptic control of neurotransmitter release (22–25). Neuronal nAChRs also are considered to modulate neurotransmitter release. In particular, there is evidence that α4-containing receptors are involved in the regulation of striatal dopamine release (26). Presynaptic 5-HT3Rs mediate an increase in intracellular Ca2+ in a subset of striatal terminals (24, 25). Hence, the enhancement of Ca2+ permeability conferred by incorporation of the nAChR α4 subunit into 5-HT3Rs might be a mechanism by which neurons can regulate Ca2+ influx at the presynaptic terminal, and thereby regulate the release of neurotransmitter. The GluR-B subunit has been shown to play an analogous role in determining the Ca2+ permeability of heteromeric AMPA receptors (27). Hippocampal interneurons express 5-HT3 and nACh receptors, which mediate rapid inward currents and presumably modulate the GABA-ergic output of these neurons (28–30). Although the exact nature of these receptors has not yet been determined, it is of interest to note that the 5-HT3Rs on interneurons in the stratum radiatum of the CA1 hippocampal area have a low sensitivity for d-tubocurarine (29). In addition, 5-HT3 and nACh receptors have been shown to mediate fast synaptic transmission in pyramidal cells and interneurons in the visual cortex of the ferret. These receptors have been suggested to be involved in the regulation of intrinsic circuit properties during development and in the adult (31). Thus, the primary condition for the formation of heteromeric receptors consisting of 5-HT3R and nAChR subunits, which is coexpression of 5-HT3 and nACh receptors in specific neuronal populations, appears to be fulfilled. However, the existence of heteromeric 5-HT3/α4 receptors in nervous tissue remains to be demonstrated.
In conclusion, this study demonstrates coassembly of authentic subunits belonging to distinct classes of neurotransmitter receptors. Previous studies have shown that within receptor families constraints on subunit interactions limit the kind of receptor species produced (32, 33). The constraints applying to coassembly of subunits from different families remain to be determined. However, promiscuous coassembly beyond the boundaries of ligand-gated ion channel families creates another level of diversity for this family of proteins.
Acknowledgments
We thank Dr. D. Julius (University of California, San Francisco), Dr. J. Patrick (Baylor College of Medicine, Houston), and Dr. W. Wisden (Division of Neurobiology, Medical Research Council Laboratory of Molecular Biology, Cambridge, United Kingdom) for the gifts of the 5-HT3R, the nAChR, and the myc-tagged GABAAR α1 subunits, respectively; Dr. J. Lindstrom (University of Pennsylvania, Philadelphia) for the sample of mAb 270; Dr. R. McKernan (Merck Sharpe & Dome, Ware, United Kingdom) for the 5-HT3R antisera; Dr. Y. Huang (National Institute on Environmental Health Sciences, Research Triangle Park, NC) for experimental support; Ms. J. Narraway and Mr. O. Verwer (Hubrecht Laboratory, Utrecht, The Netherlands) for the supply of Xenopus oocytes; Dr. D. Armstrong (National Institute on Environmental Health Sciences, Research Triangle Park, NC) for his comments on the manuscript; and Ing. A. de Groot for technical support. This work was supported in part by the Wellcome Trust (S.C.R.L.). S.C.R.L. is a Wellcome Trust Senior Research Fellow in Basic Biomedical Science.
ABBREVIATIONS
- 5-HT
serotonin (5-hydroxytryptamine)
- 5-HT3R
serotonin type 3 receptor
- BAPTA
bis(2-aminophenoxy)ethane-N, N,N′,N′-tetraacetic acid
- GABAAR
γ-aminobutyric acid type A receptor
- mCPBG
meta-chlorophenylbiguanide
- ACh
acetylcholine
- nAChR
nicotinic acetylcholine receptor
- HEK
human embryonic kidney
Note added in proof:
Recently, a paper has appeared in which the authors report that they could not detect coprecipitation of 5-HT3R and nAChR subunits from pig cerebral cortex homogenates (34). Whether promiscuous coassembly of 5-HT3R and nAChR subunits occurs in other brain areas or in small fractions of specific cell populations remains to be determined.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Maricq A V, Peterson A S, Brake A J, Myers R M, Julius D. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- 2.Hope A G, Downie D L, Sutherland L, Lambert J J, Peters J A, Burchell B. Eur J Pharmacol. 1993;245:187–192. doi: 10.1016/0922-4106(93)90128-v. [DOI] [PubMed] [Google Scholar]
- 3.Glitsch M, Wischmeyer E, Karschin A. Pflügers Arch. 1996;432:134–143. doi: 10.1007/s004240050115. [DOI] [PubMed] [Google Scholar]
- 4.Downie D L, Hope A G, Lambert J J, Peters J A, Blackburn T P, Jones B J. Neuropharmacology. 1994;33:473–482. doi: 10.1016/0028-3908(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 5.van Hooft J A, Kreikamp A P, Vijverberg H P M. J Neurochem. 1997;69:1318–1321. doi: 10.1046/j.1471-4159.1997.69031318.x. [DOI] [PubMed] [Google Scholar]
- 6.Jackson M B, Yakel J L. Annu Rev Physiol. 1995;57:447–468. doi: 10.1146/annurev.ph.57.030195.002311. [DOI] [PubMed] [Google Scholar]
- 7.Bonhaus D W, Wong E H F, Stefanich E, Kunysz E A, Eglen R M. J Neurochem. 1993;61:1927–1932. doi: 10.1111/j.1471-4159.1993.tb09835.x. [DOI] [PubMed] [Google Scholar]
- 8.Gill C H, Peters J A, Lambert J J. Br J Pharmacol. 1995;114:1211–1221. doi: 10.1111/j.1476-5381.1995.tb13335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Hooft J A, Vijverberg H P M. Eur J Pharmacol. 1997;322:229–233. doi: 10.1016/s0014-2999(96)00993-4. [DOI] [PubMed] [Google Scholar]
- 10.Eiselé J L, Bertrand S, Galzi J L, Devilliers-Thiéry A, Changeux J P, Bertrand D. Nature (London) 1993;366:479–483. doi: 10.1038/366479a0. [DOI] [PubMed] [Google Scholar]
- 11.Zwart R, Oortgiesen M, Vijverberg H P M. J Neurosci. 1995;15:2168–2178. doi: 10.1523/JNEUROSCI.15-03-02168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Hooft J A, Vijverberg H P M. Br J Pharmacol. 1996;117:839–846. doi: 10.1111/j.1476-5381.1996.tb15269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen D, Okayama H. Biotechniques. 1987;6:632–637. [PubMed] [Google Scholar]
- 14.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. pp. 61–119. [Google Scholar]
- 15.Hargreaves A C, Lummis S C R, Taylor C W. Mol Pharmacol. 1994;46:1120–1128. [PubMed] [Google Scholar]
- 16.Gilon P, Yakel J L. Receptors Channels. 1995;3:83–88. [PubMed] [Google Scholar]
- 17.Whiting P, Lindstrom J. Proc Natl Acad Sci USA. 1987;84:595–599. doi: 10.1073/pnas.84.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barish M E. J Physiol (London) 1983;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belelli D, Balcarek J M, Hope A G, Peters J A, Lambert J J, Blackburn T P. Mol Pharmacol. 1995;48:1054–1062. [PubMed] [Google Scholar]
- 20.Miyake A, Mochizuki S, Takemoto Y, Akuzawa S. Mol Pharmacol. 1995;48:407–416. [PubMed] [Google Scholar]
- 21.Yang J. J Gen Physiol. 1990;96:1177–1198. doi: 10.1085/jgp.96.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blandina P, Goldfarb J, Craddock-Royal B, Green J P. J Pharmacol Exp Ther. 1989;251:803–809. [PubMed] [Google Scholar]
- 23.Kidd E J, Laporte A M, Langlois X, Fattaccini C M, Doyen C, Lombard M C, Gozlan H, Hamon M. Brain Res. 1993;612:289–298. doi: 10.1016/0006-8993(93)91674-h. [DOI] [PubMed] [Google Scholar]
- 24.Nichols R A, Mollard P. J Neurochem. 1996;67:581–592. doi: 10.1046/j.1471-4159.1996.67020581.x. [DOI] [PubMed] [Google Scholar]
- 25.Rondé P, Nichols R A. J Neurochem. 1998;70:1094–1103. doi: 10.1046/j.1471-4159.1998.70031094.x. [DOI] [PubMed] [Google Scholar]
- 26.Wonnacott S. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- 27.Jonas P, Racca C, Sakmann B, Seeburg P H, Monyer H. Neuron. 1994;12:1281–1286. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 28.Kawa K. J Neurophysiol. 1994;71:1935–1947. doi: 10.1152/jn.1994.71.5.1935. [DOI] [PubMed] [Google Scholar]
- 29.McMahon L L, Kauer J A. J Neurophysiol. 1997;78:2493–2502. doi: 10.1152/jn.1997.78.5.2493. [DOI] [PubMed] [Google Scholar]
- 30.Jones S, Yakel J L. J Physiol (London) 1997;504:603–610. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roerig B, Nelson D A, Katz L C. J Neurosci. 1997;17:8353–8362. doi: 10.1523/JNEUROSCI.17-21-08353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vernallis A B, Conroy W G, Berg D K. Neuron. 1993;10:451–464. doi: 10.1016/0896-6273(93)90333-m. [DOI] [PubMed] [Google Scholar]
- 33.Jones A, Korpi E R, McKernan R M, Pelz R, Nusser Z, Makela R, Mellor J R, Pollard S, Bahn S, Stephenson F A, et al. J Neurosci. 1996;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fletcher S, Lindstrom J M, McKernan R M, Barnes N M. Neuropharmacology. 1998;37:397–399. doi: 10.1016/s0028-3908(98)00052-5. [DOI] [PubMed] [Google Scholar]