Abstract
Typical antipsychotic drugs, such as haloperidol and chlorpromazine, increase synthesis of the neuropeptide neurotensin (NT) in both the striatum and the nucleus accumbens, whereas atypical antipsychotic drugs, such as clozapine and olanzapine, do so only in the nucleus accumbens. By using in vivo microdialysis, we now report that acute administration of haloperidol, clozapine, or olanzapine failed to alter the release of NT in either the striatum or nucleus accumbens. In contrast, chronic administration of haloperidol for 21 days increased NT release in both the striatum and nucleus accumbens, whereas treatment for 21 days with the atypical antipsychotic drugs, clozapine or olanzapine, increased NT release selectively in the nucleus accumbens. These findings suggest that (i) increased NT mRNA expression and NT tissue concentrations are associated with increases in the extracellular fluid concentrations of the peptide and (ii) atypical antipsychotic drugs may exert their therapeutic effects and produce fewer side effects by virtue of their selectivity in limbic compared with striatal, target neurons.
The tridecapeptide neurotensin (NT) first was isolated and characterized structurally in 1973 (1). It rapidly was shown to fulfill neurotransmitter criteria, including heterogenous regional brain distribution (in a variety of mammals, including humans), calcium-dependent and depolarization-induced release, localization in synaptic ventricles, electrophysiological actions on central neurons, and the presence of high affinity NT receptors. When injected directly into the central nervous system, NT produces a number of effects strikingly similar to antipsychotic drugs, particularly the so-called atypical antipsychotics. These effects include inhibition of avoidance but not of escape, responding in a discrete trial-conditioned avoidance paradigm, blockade of psychostimulant-induced locomotor hyperactivity, and reduced rates of intracranial electrical self-stimulation (2–4). Moreover, in several studies, cerebrospinal fluid (CSF) concentrations of NT have been shown to be reduced in drug-free schizophrenic patients when compared with controls. After antipsychotic drug treatment, CSF NT concentrations increase toward normal levels (5–8). Furthermore, the administration of antipsychotic drugs in laboratory animals has been shown repeatedly to alter central nervous system NT systems (9–12). In the rat, antipsychotic drug administration results in large increases in both NT tissue concentrations and in the expression of preproneurotensin mRNA in the basal ganglia (11, 12). Of most interest, NT synthesis is altered differentially in the basal ganglia after the administration of typical vs. atypical antipsychotic drugs. Typical antipsychotics, such as haloperidol and chlorpromazine, increase NT mRNA expression and NT tissue concentrations in both the caudate nucleus and the nucleus accumbens, which are dopamine terminal regions for the nigroneostriatal and mesolimbic systems, respectively. In contrast, atypical antipsychotics, such as clozapine and olanzapine, selectively increase NT mRNA expression and NT tissue concentrations in the nucleus accumbens (2, 9, 10, 12). Whether antipsychotic drug-induced increases in NT content and NT mRNA expression are associated with increases in NT release into the extracellular fluid remains unknown. This question remains unanswered not only for NT but for other neuropeptides as well.
There is compelling evidence that the extrapyramidal side effects (EPSEs) associated with typical antipsychotic drugs are caused by effects on the nigroneostriatal dopamine pathway (13). These drugs have been available for >40 years, and, although effective for the positive symptoms of schizophrenia (e.g., psychosis, auditory hallucinations, etc.), they do have a propensity to cause EPSEs, including acute dystonic reactions and tardive dyskinesia. In contrast, atypical antipsychotic drugs appear to act selectively on the mesolimbocortical dopamine system (14). These atypical antipsychotic drugs cause no EPSEs and, more importantly, appear to be more effective both in treating the negative symptoms of schizophrenia (e.g., lack of affect) and in managing treatment-resistant schizophrenic patients (14). Our group and others have hypothesized that differences in the effects of typical vs. atypical antipsychotic drugs on NT neurons after chronic administration of antipsychotic drugs may underlie the clinical differences between these classes of agents.
The purpose of the present series of experiments was twofold. First, we sought to determine, by using microdialysis, whether the antipsychotic drug-induced increase in NT mRNA expression and NT concentrations in the central nervous system are associated with increases in extracellular fluid concentrations of NT. Second, we sought to determine, by using this technique, whether differential effects of prototype-atypical antipsychotics (olanzapine and clozapine) could be distinguished from a prototype-typical antipsychotic (haloperidol).
METHODS
Microdialysis.
The microdialysis probes were constructed in a loop design to enhance the surface area of the exposed semipermeable membrane (polyaminonitrile; Spectrum Laboratories, Houston) and were placed stereotaxically in anesthetized (ketamine 37.5 mg⋅kg−1, xylamine 50 mg⋅kg−1, and acepromazine 1.5 mg⋅kg−1) male Sprague–Dawley rats (275–325 g, Charles River Breeding Laboratories) in both the striatum (AP + 0.2, ML ± 2.5, V-7.0) and the nucleus accumbens (AP + 1.2, ML ± 1.5, V-8.0). All experiments were approved by the Institute of Animal Care and Use Committee at Emory University, in accordance with Public Health Service guidelines. The probes were secured to the skull by using dental cement, and the animal was allowed to recover overnight in a microdialysis setup (CMA/Carnegie Medicin, Stockholm) that was attached to a pump using phosphatidylethanolamine 10 tubing. Artificial CSF [125 mM CaCl2/2.5 mM KCl/2.2 mM NaH2PO4/0.2 mM Na2HPO4/1.2 mM CaCl2/1.0 mM MgCl2/0.2 mM ascorbic acid/0.025% (wt/vol) BSA, pH 7.2] was perfused through the probes at a flow rate of 1.0 μl⋅min−1 (striatum) or 0.5 μl⋅min−1 (nucleus accumbens). For samples containing 100 mM KCl, the concentration of NaCl was reduced to 27.5 mM. Similarly, artificial CSF containing 0 mM CaCl2 and 2.2 mM MgCl2 was used. Samples were collected over 30 (striatum) or 60 mins (nucleus accumbens) and were stored at −70°C until assayed.
Radioimmunoassay.
NT-like immunoreactivity was measured by using a solid-phase radioimmunoassay. Nunc immunomodule plates (Maxisorb) were coated with protein G (250 ng/well−1), allowing for the NT-specific antibody (carboxyl-terminal) to attach to the wells. A standard curve was performed by using standards and/or samples containing NT in competition with 125I-Tyr1-NT over a 24-hr incubation period. Measurements of NT concentrations of 0.2–25 picograms are done routinely by using this procedure (7, 12).
Drug Administration: Effects of Acute Administration of Antipsychotic Drugs on NT Release.
Animals were implanted with microdialysis probes as described in ref. 8, and the following day, three basal samples were collected, followed by an injection of haloperidol (2.0 mg⋅kg−1 s.c.), clozapine (20 mg⋅kg−1 s.c.), or vehicle [0.3% (wt/vol) tartaric acid, 1.0 ml⋅kg−1]. After the injection, samples were collected hourly for 26 hr. At the end of the experiment, the artificial CSF was switched to CSF containing 100 mM KCl to test for neuronal viability. Animals were implanted with osmotic pumps (Model 2ML4, Alza) for the continuous infusion of one of the following: vehicle [0.3% (wt/vol) tartaric acid], haloperidol (2.0 mg⋅kg−1⋅day−1), clozapine (40.0 mg⋅kg−1⋅day−1), or olanzapine (20.0 mg⋅kg−1⋅day−1). Twenty-one days after the implantation, NT release was examined in awake, freely moving rats. Four samples were collected from each animal on day 21 of treatment, and blood was obtained for measurement of drug concentration. The average of the four samples was used as the NT concentration of each animal, and mean values per group were compared with their own vehicle control group’s value by using the Student’s t test. Thus, separate control groups were used for each drug treatment and for both brain regions studied. Plasma drug concentrations were measured as described elsewhere (16–18) to ensure plasma concentrations that were comparable to the clinical setting (13, 14).
RESULTS AND DISCUSSION
The effects of typical and atypical antipsychotic drugs on NT release in the awake, freely moving rat were examined. In vivo microdialysis, in combination with a very sensitive (≥0.2 picograms) solid-phase radioimmunoassay technique, was used to measure extracellular fluid NT concentrations in the rat brain (15). Initial studies were performed to establish the neuronal origin of the NT measured in the dialysate. To test these findings, five basal samples were collected from each region; sample six represented the sample containing artificial CSF with 100 mM KCl. The exposure of 100 mM KCl resulted in a significant increase in NT release in both the striatum [F (5, 45) = 3.17, P < 0.01] and the nucleus accumbens [F (4, 36) = 10.1, P < 0.01] (data not shown). To characterize this release further, additional experiments were performed by using artificial CSF in the absence of CaCl2, and the removal of calcium completely inhibited the 100- mM, KCl-induced increase in NT release.
Once in vivo NT release had been characterized, the effects of acute administration of antipsychotic drugs, both typical (haloperidol) and atypical (clozapine, olanzapine), on NT release were studied. Because NT tissue concentrations have been shown to increase maximally ≈12–18 hr after acute administration of haloperidol and because NT mRNA expression increases 6–12 hr after injection, it was important to examine NT release over an extended period of time (30 hr). A single injection of haloperidol resulted in no significant changes in NT release over the 26 hr after injection in either the striatum or the nucleus accumbens. Similarly, acute administration of the atypical antipsychotic drugs, clozapine (20.0 mg⋅kg−1 s.c.) and olanzapine (1.0 mg⋅kg−1, 10.0 mg⋅kg−1 s.c.), had no effect on NT release in either region.
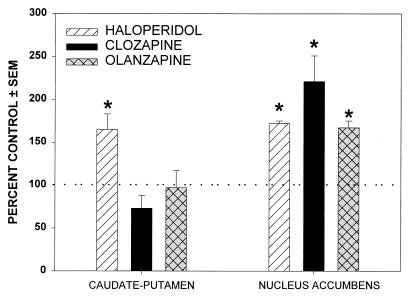
To determine the effects of chronic administration of antipsychotic drugs on in vivo NT release, rats were implanted with 28-day osmotic mini-pumps (Model 2ML4, Alza) with haloperidol (2.0 mg⋅kg−1⋅day−1), clozapine (40.0 mg⋅kg−1⋅day−1), or olanzapine (20.0 mg⋅kg−1⋅day−1), as well as with vehicle control (0.3% wt/vol tartaric acid). On day 20, animals were implanted with microdialysis probes and were allowed to recover overnight, and samples were collected from both the striatum (1.0 μl⋅min−1 × 30 min) and the nucleus accumbens (0.5 μl⋅min−1 × 60 min) the following day. As shown in Fig. 1, chronic administration of haloperidol significantly increased the basal NT release in both the caudate nucleus and the nucleus accumbens. In contrast, chronic administration of either clozapine or olanzapine increased NT release only in the nucleus accumbens. To ensure that the dosages used were comparable with the clinical setting, plasma drug concentrations were measured to attain plasma levels of the drugs comparable to those of patients who respond to these agents clinically (16, 17).
Figure 1.
Effects of chronic (21-day) administration of antipsychotic drugs on NT release. Values are expressed as a percentage of the control group for graphic representation. Basal values (expressed as picograms of NT per hour±SEM) of the vehicle control group for each of the three major experiments were: A. haloperidol, striatum: 10.98 ± 3.40 (n = 7); nucleus accumbens: 4.34 ± 0.69 (n = 6); B. clozapine, striatum: 10.36 ± 0.80 (n = 5); nucleus accumbens: 2.94 ± 0.40 (n = 5); C. olanzapine, striatum: 8.85 ± 0.60 (n = 6); nucleus accumbens: 2.15 ± 0.03 (n = 6). Haloperidol increased NT release in both the caudate-putamen and the nucleus accumbens. Olanzapine and clozapine increased NT release only in the nucleus accumbens.
The antipsychotic drug-induced increases in in vivo NT release demonstrated in these experiments support our hypothesis that the antipsychotic drug-induced increases in both NT tissue concentrations and NT mRNA expression increase the synaptic availability of the peptide. These effects were observed after chronic, but not acute, antipsychotic drug administration. These findings are also concordant with the view that these changes in NT availability may underlie, in part, the therapeutic actions of antipsychotic drugs. It is well established that these agents typically require at least 2 wk of administration before they are clinically effective (13, 14). The exact locus of action of antipsychotic drugs in altering NT mRNA expression and NT synthesis and release remains obscure. Multiple interactions of NT and dopamine systems have been demonstrated both in the midbrain (substantia nigra and ventral tegmental area) and in the forebrain (nucleus accumbens and caudate nucleus). The fact that antipsychotic drugs added to in vitro preparations of primary neuronal cultures of striatal and accumbens increase NT synthesis sharply suggests NT neurons in these areas as direct targets of antipsychotic drugs in mediating the observed effects (C. D. Kilts, P. Lambert and C.B.N., unpublished observations).
The changes we observed after chronic administration of antipsychotic drugs have other potentially important clinical implications. The observation that chronic administration of haloperidol increases NT release in the caudate nucleus is concordant with the hypothesis that changes in the functioning of NT-containing neurons in these regions may play a role in the development of EPSEs. The atypical antipsychotic drugs (clozapine and olanzapine), in contrast to haloperidol, do not alter striatal NT release but, like the typical antipsychotics, increase NT release in the nucleus accumbens. These findings, taken together with previous observations (2, 9–12), suggest that increases in NT transmission in the nucleus accumbens are predictive of the clinical efficacy of putative antipsychotic drugs, whereas increases in NT neurotransmission in the striatum are predictive of EPSEs.
The implications of this work both for the neurobiology of neuropeptide neurons and for the mechanisms of action of antipsychotic drugs may be important. For the former, it is clear that, with a sufficiently sensitive assay method, detection of changes in neuropeptide concentration in extracellular fluid can be detected in response to pharmacological or physiological perturbations. For the latter, the present findings may be important for new drug development. If an increase in NT concentration in extracelluar fluid in specific brain regions is associated with response to atypical antipsychotic drugs, then specific NT receptor agonists may represent a new class of atypical antipsychotic agents.
Acknowledgments
We are grateful to Dr. Glen Hanson, Department of Pharmacology and Toxicology, University of Utah, Salt Lake City, for his technical advice in developing the radioimmunoassay. This work was supported by National Institute of Mental Health Grant MH-39415.
ABBREVIATIONS
- NT
neurotensin
- CSF
cerebrospinal fluid
- EPSEs
extrapyramidal side effects
References
- 1.Carraway R E, Leeman S E. J Biol Chem. 1973;248:6854–6861. [PubMed] [Google Scholar]
- 2.Nemeroff C B, Levant B, Myers B, Bissette G. Ann N Y Acad Sci. 1992;668:146–156. doi: 10.1111/j.1749-6632.1992.tb27346.x. [DOI] [PubMed] [Google Scholar]
- 3.Ford A P D W, Marsden C A. Brain Res. 1990;534:243–250. doi: 10.1016/0006-8993(90)90135-x. [DOI] [PubMed] [Google Scholar]
- 4.Jolicoeur F B, Gagne M A, Rivest R, Drumheller A, St.-Pierre S. Brain Res Bull. 1993;32:487–491. doi: 10.1016/0361-9230(93)90295-m. [DOI] [PubMed] [Google Scholar]
- 5.Breslin N A, Suddath R L, Bissette G, Nemeroff C B, Lowrimore P, Weinberger D R. Schizophr Res. 1994;12:35–41. doi: 10.1016/0920-9964(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 6.Widerlov E, Lindstrom L, Besev G, Manberg P J, Nemeroff C B, Breese G R, Kizer J S, Prange A J. Am J Psychiatry. 1982;139:1122–1126. doi: 10.1176/ajp.139.9.1122. [DOI] [PubMed] [Google Scholar]
- 7.Garver D L, Bissette G, Yao J K, Nemeroff C B. Am J Psychiatry. 1991;148:484–488. doi: 10.1176/ajp.148.4.484. [DOI] [PubMed] [Google Scholar]
- 8.Sharma R P, Janicak P G, Bissette G, Nemeroff C B. Am J Psychiatry. 1997;154:1019–1021. doi: 10.1176/ajp.154.7.1019. [DOI] [PubMed] [Google Scholar]
- 9.Kilts C D, Anderson C M, Bissette G, Ely T D, Nemeroff C B. Biochem Pharmacol. 1988;37:1547–1554. doi: 10.1016/0006-2952(88)90017-2. [DOI] [PubMed] [Google Scholar]
- 10.Kinkead B, Nemeroff C B. J Clin Psychiatry. 1994;55:30–32. [PubMed] [Google Scholar]
- 11.Levant B, Merchant K M, Dorsa D M, Nemeroff C B. Mol Brain Res. 1992;12:279–284. doi: 10.1016/0169-328x(92)90130-4. [DOI] [PubMed] [Google Scholar]
- 12.Merchant K M, Dobie D J, Filloux F M, Totzke M, Aravagiri M, Dorsa D M. J Pharmacol Exp Ther. 1994;271:460–471. [PubMed] [Google Scholar]
- 13.Marder S R, Van Putten T. In: Textbook of Psychopharmacology. Schatzberg A F, Nemeroff C B, editors. Washington, DC: American Psychiatric Press; 1995. pp. 247–261. [Google Scholar]
- 14.Owens M J, Risch S C. In: Textbook of Psychopharmacology. Schatzberg A F, Nemeroff C B, editors. Washington, DC: American Psychiatric Press; 1995. pp. 263–280. [Google Scholar]
- 15.Wagstaff J D, Gibb J W, Hanson G R. Brain Res. 1996;721:196–203. doi: 10.1016/0006-8993(96)00132-1. [DOI] [PubMed] [Google Scholar]
- 16.Mazher M, Bender J. J Chromatogr. 1989;497:201–212. doi: 10.1016/0378-4347(89)80019-2. [DOI] [PubMed] [Google Scholar]
- 17.Ritchie J C, Zhang W. Clin Chem. 1996;42:5222. (abstr.). [Google Scholar]
- 18.Chiu J A, Franklin R B. J Pharm Biomed Anal. 1996;14:609–615. doi: 10.1016/0731-7085(95)01651-1. [DOI] [PubMed] [Google Scholar]