Abstract
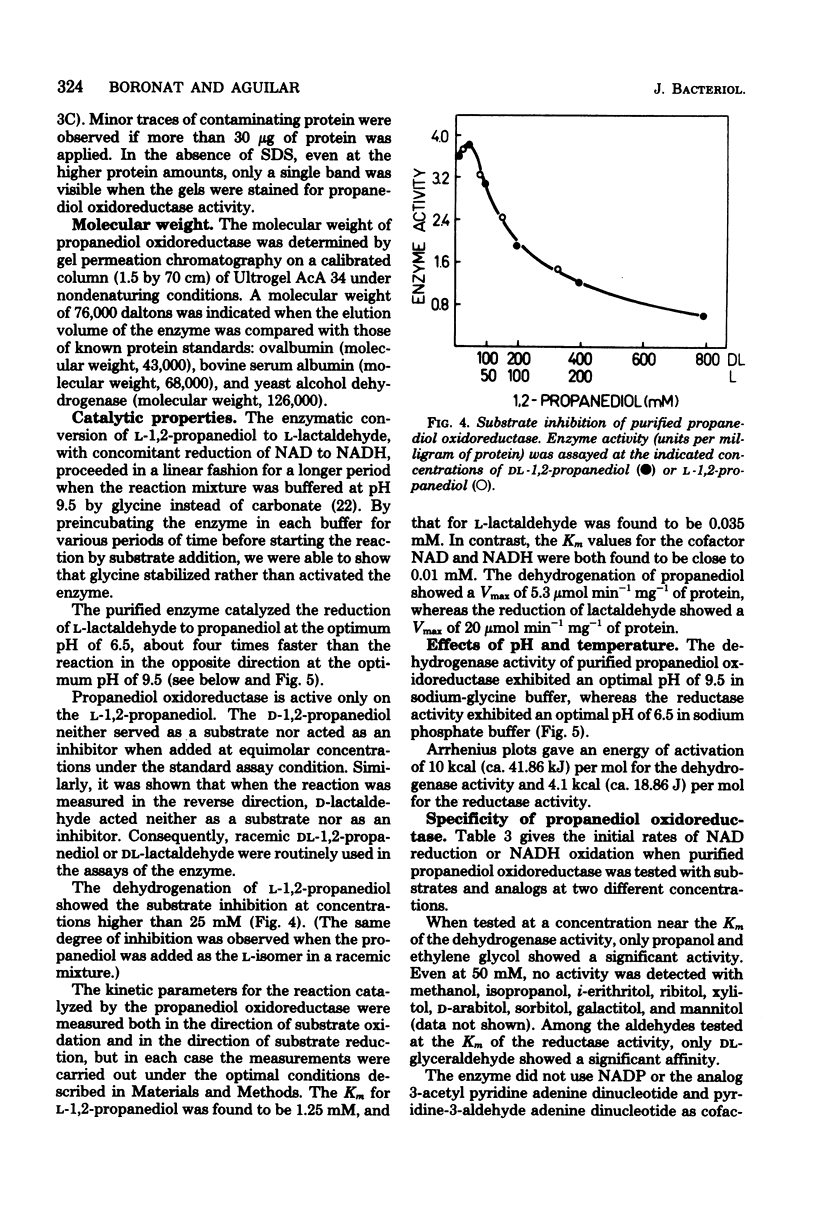
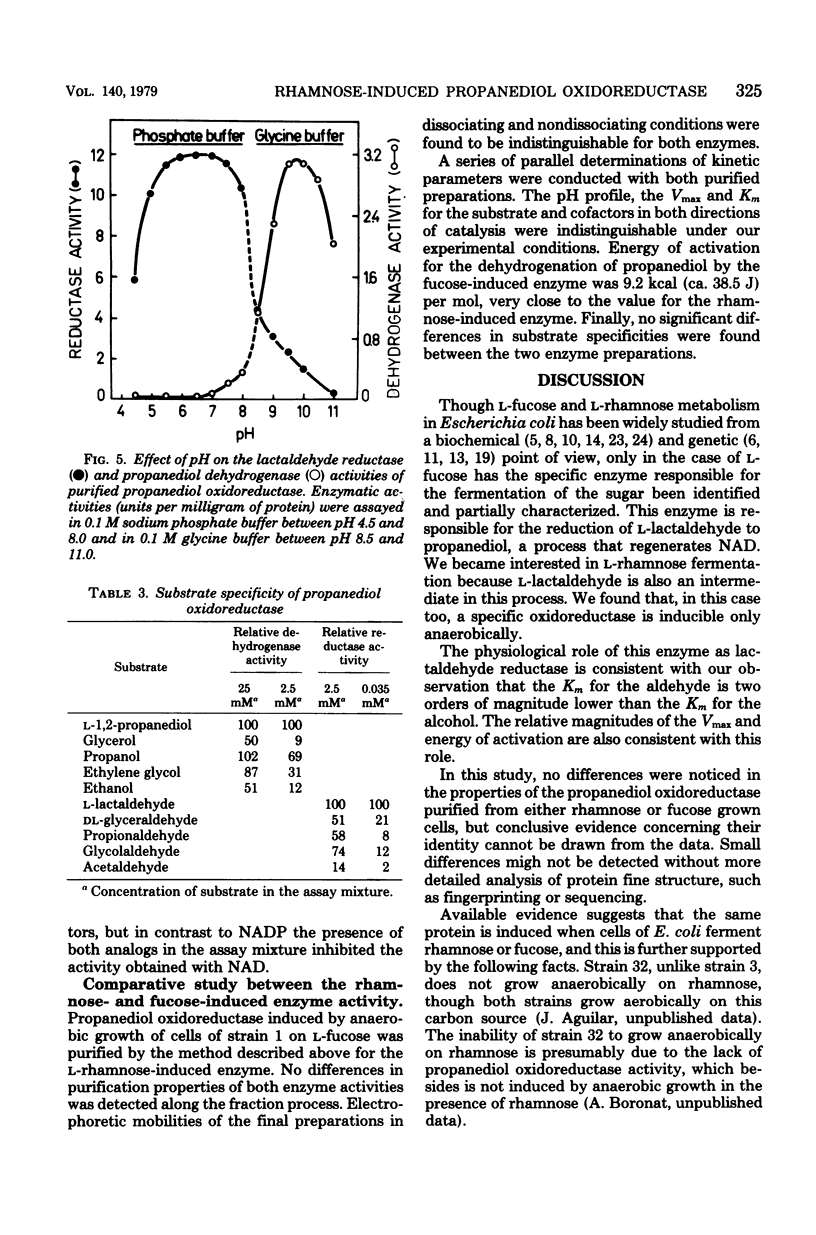
Escherichia coli are capable of growing anaerobically on L-rhamnose as a sole source of carbon and energy and without any exogenous hydrogen acceptor. When grown under such condition, synthesis of a nicotinamide adenine dinucleotide-linked L-lactaldehydepropanediol oxidoreductase is induced. The functioning of this enzyme results in the regeneration of nicotinamide adenine dinucleotide. The enzyme was purified to electrophoretic homogeneity. It has a molecular weight of 76,000, with two subunits that are indistinguishable by electrophoretic mobility. The enzyme reduces L-lactaldehyde to L-1,2-propanediol with reduced nicotinamide adenine dinucleotide as a cofactor. The Km were 0.035 mM L-lactaldehyde and 1.25 mM L-1,2-propanediol, at pH 7.0 and 9.5, respectively. The enzyme acts only on the L-isomers. Strong substrate inhibition was observed with L-1,2-propanediol (above 25 mM) in the dehydrogenase reaction. The enzyme has a pH optimum of 6.5 for the reduction of L-lactaldehyde and of 9.5 for the dehydrogenation of L-1,2-propanediol. The enzyme is, according to the parameters presented in this report, indistinguishable from the propanediol oxidoreductase induced by anaerobic growth on fucose.
Full text
PDF
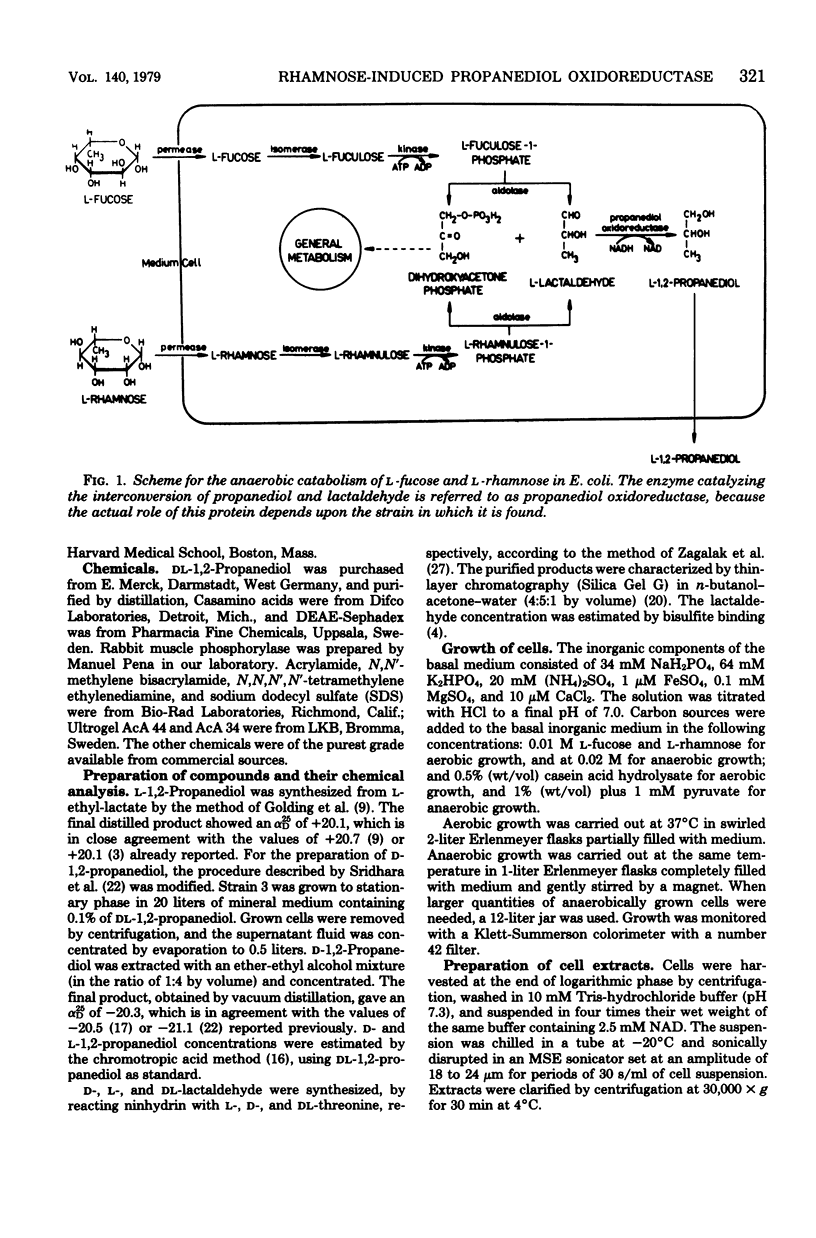
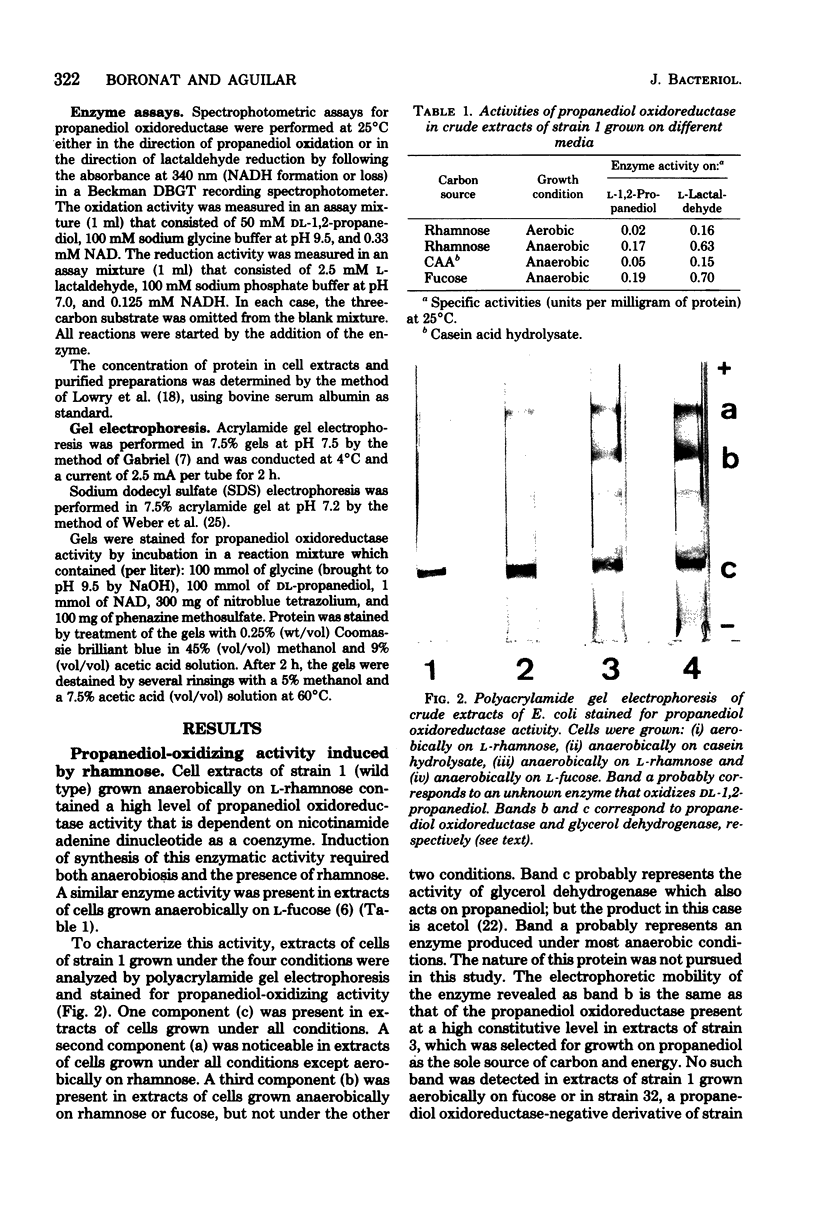
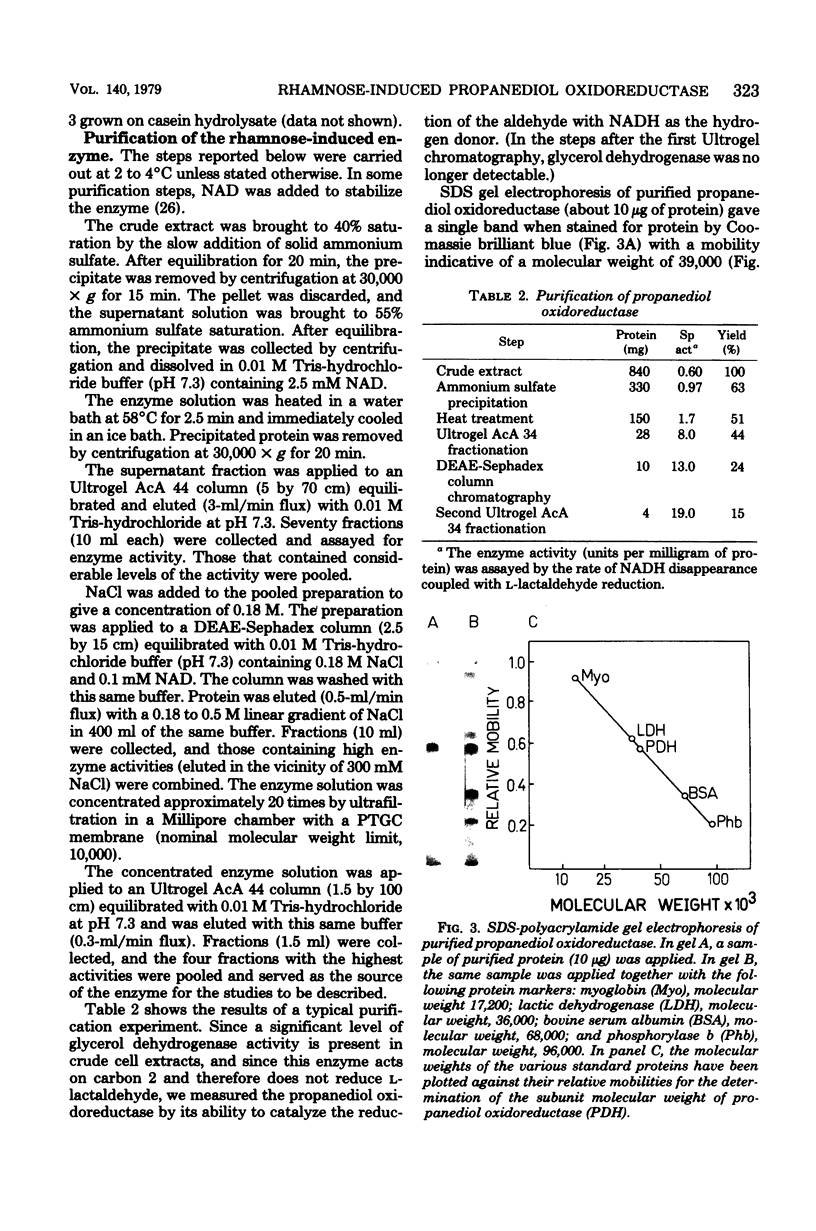

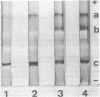
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu T. H., Feingold D. S. L-rhamnulose 1-phosphate aldolase from Escherichia coli. Crystallization and properties. Biochemistry. 1969 Jan;8(1):98–108. doi: 10.1021/bi00829a015. [DOI] [PubMed] [Google Scholar]
- Cocks G. T., Aguilar T., Lin E. C. Evolution of L-1, 2-propanediol catabolism in Escherichia coli by recruitment of enzymes for L-fucose and L-lactate metabolism. J Bacteriol. 1974 Apr;118(1):83–88. doi: 10.1128/jb.118.1.83-88.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHALAMBOR M. A., HEATH E. C. The metabolism of L-fucose. II. The enzymatic cleavage of L-fuculose 1-phosphate. J Biol Chem. 1962 Aug;237:2427–2433. [PubMed] [Google Scholar]
- GREEN M., COHEN S. S. Enzymatic conversion of L-fucose to L-fuculose. J Biol Chem. 1956 Apr;219(2):557–568. [PubMed] [Google Scholar]
- HEATH E. C., GHALAMBOR M. A. The metabolism of L-fucose. I. The purification and properties of L-fuculose kinase. J Biol Chem. 1962 Aug;237:2423–2426. [PubMed] [Google Scholar]
- Hacking A. J., Aguilar J., Lin E. C. Evolution of propanediol utilization in Escherichia coli: mutant with improved substrate-scavenging power. J Bacteriol. 1978 Nov;136(2):522–530. doi: 10.1128/jb.136.2.522-530.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacking A. J., Lin E. C. Disruption of the fucose pathway as a consequence of genetic adaptation to propanediol as a carbon source in Escherichia coli. J Bacteriol. 1976 Jun;126(3):1166–1172. doi: 10.1128/jb.126.3.1166-1172.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacking A. J., Lin E. C. Regulatory changes in the fucose system associated with the evolution of a catabolic pathway for propanediol in Escherichia coli. J Bacteriol. 1977 May;130(2):832–838. doi: 10.1128/jb.130.2.832-838.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORN E. D. Clearing factor, a heparin-activated lipoprotein lipase. I. Isolation and characterization of the enzyme from normal rat heart. J Biol Chem. 1955 Jul;215(1):1–14. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Power J. The L-rhamnose genetic system in Escherichia coli K-12. Genetics. 1967 Mar;55(3):557–568. doi: 10.1093/genetics/55.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhara S., Wu T. T., Chused T. M., Lin E. C. Ferrous-activated nicotinamide adenine dinucleotide-linked dehydrogenase from a mutant of Escherichia coli capable of growth on 1, 2-propanediol. J Bacteriol. 1969 Apr;98(1):87–95. doi: 10.1128/jb.98.1.87-95.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhara S., Wu T. T. Purification and properties of lactaldehyde dehydrogenase from Escherichia coli. J Biol Chem. 1969 Oct 10;244(19):5233–5238. [PubMed] [Google Scholar]
- TAKAGI Y., SAWADA H. THE METABOLISM OF L-RHAMNOSE IN ESCHERICHIA COLI. I. L-RHAMNOSE ISOMERASE. Biochim Biophys Acta. 1964 Oct 23;92:10–17. doi: 10.1016/0926-6569(64)90263-9. [DOI] [PubMed] [Google Scholar]
- TAKAGI Y., SAWADA H. THE METABOLISM OF L-RHAMNOSE IN ESCHERICHIA COLI. II. L-RHAMNULOSE KINASE. Biochim Biophys Acta. 1964 Oct 23;92:18–25. doi: 10.1016/0926-6569(64)90264-0. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Wu T. T. Growth of a mutant of Escherichia coli K-12 on xylitol by recruiting enzymes for D-xylose and L1,2-propanediol metabolism. Biochim Biophys Acta. 1976 May 28;428(3):656–663. doi: 10.1016/0304-4165(76)90195-1. [DOI] [PubMed] [Google Scholar]
- Zagalak B., Frey P. A., Karabatsos G. L., Abeles R. H. The stereochemistry of the conversion of D and L 1,2-propanediols to propionaldehyde. J Biol Chem. 1966 Jul 10;241(13):3028–3035. [PubMed] [Google Scholar]