Abstract
Evidence suggests that cholinergic input to the hippocampus plays an important role in learning and memory and that degeneration of cholinergic terminals in the hippocampus may contribute to the memory loss associated with Alzheimer’s disease. One of the more prominent effects of cholinergic agonists on hippocampal physiology is the potentiation of N-methyl-d-aspartate (NMDA)-receptor currents by muscarinic agonists. Here, we employ traditional pharmacological reagents as well as m1-toxin, an m1 antagonist with unprecedented selectivity, to demonstrate that this potentiation of NMDA-receptor currents in hippocampal CA1 pyramidal cells is mediated by the genetically defined m1 muscarinic receptor. Furthermore, we demonstrate the colocalization of the m1 muscarinic receptor and the NR1a NMDA receptor subunit at the electron microscopic level, indicating a spatial relationship that would allow for physiological interactions between these two receptors. This work demonstrates that the m1-muscarinic receptor gene product modulates excitatory synaptic transmission, and it has important implications in the study of learning and memory as well as the design of drugs to treat neurodegenerative diseases such as Alzheimer’s.
One of the major neuromodulatory inputs to the hippocampus is a large cholinergic projection from the medial septum and the diagonal band of Broca (1). Both animal and human studies indicate that cholinergic modulation of hippocampal and cortical function plays an important role in memory and attention (2–7). Furthermore, abundant evidence suggests that the clinical syndrome associated with Alzheimer’s disease results, at least in part, from the degeneration of basal forebrain cholinergic neurons and the resulting depletion of cholinergic markers in neocortex and hippocampus (8–12). Because of this, a great deal of effort has been focused on determining the cellular mechanisms involved in cholinergic modulation of hippocampal function and the specific acetylcholine (ACh) receptor subtypes that mediate these responses.
One of the predominant effects of cholinergic agonists on hippocampal CA1 neurons is potentiation of currents through the N-methyl-d-aspartate (NMDA) subtype of glutamate receptor (NMDAR) (13–16). The NMDAR plays a pivotal role in long-lasting forms of synaptic plasticity thought to underlie learning and memory (17). Thus, potentiation of NMDAR currents (INMDA) could provide a crucial mechanism by which cholinergic input to the hippocampus modulates memory and attention. In addition, the cholinergic receptor that mediates this potentiation could provide a target for the development of drugs to treat memory disorders (e.g., Alzheimer’s disease). Evidence suggests that ACh-induced potentiation of NMDAR currents is mediated by muscarinic ACh receptors (mAChRs) (14). However, the specific mAChR subtype that mediates this response is not known. The mAChRs have been classified into m1–m5 subtypes based on molecular analysis of genes that encode five highly related but structurally distinct mAChR subtypes (18–20). Using a panel of highly specific antibodies for each of the mAChR subtypes, we detected three of the five mAChRs (m1, m3, and m4) in CA1 pyramidal cells where they could mediate mAChR-induced potentiation of NMDAR currents (21). Of these subtypes, m1 is by far the most abundant in the hippocampal formation, and this receptor is especially enriched in hippocampal area CA1 (21, 22). Furthermore, ultrastructural analysis of m1 immunoreactivity reveals that this receptor is heavily localized in spines and dendrites of asymmetric (putative glutamatergic) synapses in the cerebral cortex (23) and hippocampus (24). Finally, Markram and Segal (15) provided evidence that the potentiation of NMDAR currents by mAChR agonists requires activation of phosphoinositide hydrolysis. Of the three candidate receptors, only m1 and m3 couple to this effector system (19). Based on these previous studies, we postulate that mAChR-induced potentiation of NMDAR currents in CA1 pyramidal cells is mediated by m1.
In the present studies, we employ a combination of competitive mAChR antagonists and m1-toxin, a highly specific and irreversible m1 antagonist (25–28) to demonstrate that the carbachol (CCh)-induced potentiation of NMDAR currents is mediated by an m1-like receptor. We also utilize receptor subtype and subunit-specific antibodies for double-labeling immunocytochemistry to demonstrate that m1 and the NR1a NMDAR subunit are colocalized at specific postsynaptic sites. This reveals a spatial relationship that would allow for specific physiological interactions between these two receptor subtypes. These studies provide conclusive evidence that the m1-muscarinic receptor gene product mediates the modulation of NMDAR-mediated excitatory synaptic transmission and provide a possible mechanism by which m1 agonists may be effective in ameliorating the cognitive deficits associated with Alzheimer’s disease.
MATERIALS AND METHODS
Materials.
Male Sprague–Dawley rats were used for all experiments. In rats, the adult expression profile of the m1 receptor is fully developed by 3 weeks postnatal (29), and the NR1 receptor is expressed at high levels throughout the brain beginning at birth (30). Therefore, we used rats that were at least 3 weeks old for all experiments. PD 102807 was a gift from Roy Schwarz (Parke-Davis). m1-toxin was previously purified and characterized in the laboratory of L.T.P. (25). 1S,3R-ACPD and NMDA were obtained from (Tocris Neuramin, Bristol, U.K.). All other materials were obtained from Sigma.
Electrophysiology.
For patch-clamp studies, hippocampi from 3- to 6-week-old (101–125 g) male Sprague–Dawley rats were dissected on ice. Thick (400-μm) transverse hippocampal slices were prepared and maintained as described previously (31). Slices were maintained fully submerged on the stage of a brain slice chamber and perfused continuously with artificial cerebrospinal fluid (ACSF) (1 ml/min, equilibrated with 95% O2/5% CO2). Patch electrodes were pulled from borosilicate glass on a Narashige vertical patch-pipette puller and filled with 40 mM Hepes/100 mM gluconic acid/0.6 mM EGTA/0.3 mM GTP/2 mM ATP/5 mM MgCl2; pH was adjusted to 7.4 with 50% CsOH. Electrode resistance was 3–7 MΩ. The patch electrode was advanced through the air/ACSF interface and into the pyramidal cell layer of area CA1 while maintaining slight positive pressure, and recordings from CA1 pyramidal cells were made by using the “blind” patch/slice technique. For measurement of NMDA-evoked currents NMDA was pressure-ejected into the slice from a low-resistance pipette. NMDA-evoked currents were recorded from a holding potential of −60 mV, and slices were bathed in ACSF containing 1 μM tetrodotoxin (Sigma). Percent potentiation was defined by using the ratio of maximum current amplitude during carbachol application (Imax) to average current amplitude of three trials immediately preceding drug application (Ibase) in the equation: percent potentiation = {[(Imax/Ibase) − 1] × 100}. For measurement of stimulus-evoked postsynaptic currents in area CA1, bipolar tungsten electrodes were used to apply stimuli to the Schaffer collateral–commissural pathway. In these studies, the bathing ACSF contained 50 μM picrotoxin, and the Schaffer collateral pathway was cut at the CA1/CA3 border to eliminate excitatory input from area CA3. Drugs were applied through the perfusion medium. An IBM Pentium clone and pclamp data acquisition and analysis system (Axon Instruments) were used to acquire and analyze the data. All data are presented as means ± SEM.
Immunocytochemistry.
Sprague–Dawley rats (250–300 g) were deeply anesthetized with 4% chloral hydrate, then perfused transcardially with a phosphate-buffered solution of 3% paraformaldehyde and 0.15 or 0.2% glutaraldehyde for 12–13 min (260–290 ml). The rats then were refrigerated for 1–2 hr, after which their brains were removed and 40- to 50-μm sections of the dorsal hippocampus were obtained on a vibratome (Oxford). Sections subsequently were preblocked with 4% normal goat serum (NGS) in Tris-buffered saline (TBS) (50 mM Tris/1.5% NaCl, pH 7.2) and then incubated in primary NMDA mAb (1:200) (mAb 54.1, PharMingen) and m1 rabbit polyclonal affinity-purified antibody (1 μg/ml) in TBS containing 2% normal goat serum for at least 48 hr. The generation and detailed characterization of the specificity of these antibodies have been described previously (22–34). Control sections were treated identically, except they received only one of the two primary antibodies or no primary antibody. After several rinses in TBS, the sections were incubated in the following secondary antibodies for 24 hr: goat anti-mouse (for monoclonal NMDA) and gold-conjugated goat anti-rabbit (for polyclonal m1). The NMDAR monoclonal immunoreactivity was amplified with mouse peroxidase antiperoxidase (PAP) and developed in diaminobenzidine (DAB, Sigma) containing 0.5% hydrogen peroxide, rinsed in TBS followed by a phosphate buffer rinse, and then postfixed overnight in 4% glutaraldehyde in phosphate buffer. Sections were then rinsed thoroughly in 0.1 M phosphate buffer (pH 7.6), processed with a silver enhancement protocol as described in Burry et al. (35), and postfixed in 1% osmium tetroxide in phosphate buffer for 1 hr. Sections then were dehydrated through a graded ethanol series, followed by propylene oxide, infiltrated, and embedded in Eponate 12 (Ted Pella, Redding, CA) between glass slides coated with releasing agent. Silver and silver-gold sections were obtained by using a diamond knife on a Reichert Ultracut S (Leica) ultramicrotome. Sections were retrieved on 200 mesh hexagonal grids, viewed, and photographed on a JEOL 100 C electron microscope. Profiles were considered immunoreactive if they contained the diffuse electron-dense DAB reaction product (marking NR1a immunoreactivity) and/or three or more gold particles (marking m1 immunoreactivity), within the membrane boundaries of that profile.
RESULTS
Bath Application of Carbachol Induces a Rapidly Desensitizing Potentiation of NMDA Receptor Currents in CA1 Pyramidal Cells.
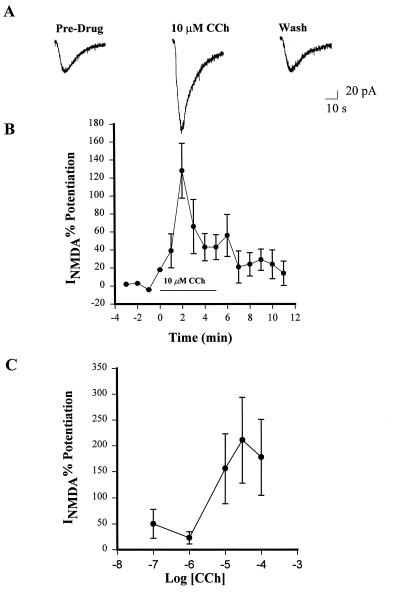
NMDA (1 mM) applied to stratum radiatum at 1-min intervals by pressure ejection produced a stable inward current (30–100 pA) in CA1 pyramidal cells held at −60 mV (INMDA). Bath application of 10 μM CCh produced an approximate 2-fold increase in INMDA amplitude (Fig. 1A and B). This potentiation peaked within 2–3 min of the start of CCh infusion and was followed by a decay to baseline (Fig. 1A). After 5 min of washing, there was no significant difference between the pre and postdrug currents. In a few experiments CCh was reapplied after a 5- to 10-min washout period. In these cases, the potentiating effect of CCh was diminished greatly, indicating that desensitization had occurred. Therefore, we limited all experiments to a single drug application. The effect exhibited a dose dependency with an approximate EC50 of 10 μM (Fig. 1C). This is consistent with the EC50 for carbachol-induced stimulation of phosphoinositide hydrolysis in brain slices (36–38).
Figure 1.
Activation of muscarinic receptors potentiates NMDA-receptor currents in CA1 pyramidal cells. Bath application of 10 μM CCh induces a marked potentiation of currents evoked by NMDA application. (A) Single traces obtained before CCh application (Pre-Drug), at the peak of CCh-induced potentiation (10 μM CCh), and after a 5-min washout. (B) The time course of CCh-induced potentiation of NMDAR currents demonstrates that this effect quickly desensitizes. Data represent mean ± SEM of five cells. (C) The potentiating effect of CCh is dose-dependent in the range of 1–100 μM. Data represent mean ± SEM of peak potentiation with three cells for each data point.
The Effect of mAChR Antagonists on CCh-Induced Potentiation of NMDA-Receptor Currents Is Consistent with Mediation by m1.
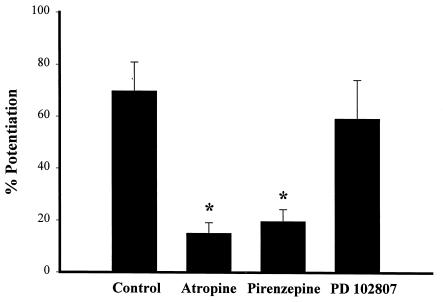
We employed the nonselective antagonist atropine to verify that the effect of CCh was mediated by mAChR activation. Atropine (1 μM) reduced CCh-induced potentiation of INMDA by 78.4% compared with control cells (Fig. 2), indicating that this effect is mediated by mAChRs. We next determined the effect of pirenzepine, an mAChR antagonist with selectivity for m1 relative to other mAChR subtypes. Pirenzepine (75 nM) significantly reduced the CCh induced potentiation of INMDA at a concentration approximately 10 times its IC50 at m1 receptors (Fig. 2). However, while pirenzepine exhibits more than 20-fold selectivity for m1 relative to m2 or m3, this compound is only about 5-fold selective for m1 over m4 (39). Thus, an effect of this concentration of pirenzepine is consistent with mediation by either m1 or the m4 receptor. To determine whether m4 might mediate this response to CCh, we employed the m4-selective antagonist PD102807 (40, 41). PD102807 is 100-fold more potent at m4 than at m1 receptors. Cells pretreated with 250 nM PD102807, a concentration approximately 10 times its IC50 at m4 receptors, exhibited no significant difference in response to CCh (Fig. 2). Thus, the inhibitory effect of pirenzepine is unlikely to be mediated by m4.
Figure 2.
The effect of mAChR antagonists on the CCh-induced potentiation of NMDA-receptor currents. Data presented show the effect of CCh (10 μM) on peak NMDAR currents in the absence of antagonists (Control) or in the presence of the nonspecific mAChR antagonist atropine (1 μM), the m1-selective antagonist pirenzepine (75nM), or the m4-selective antagonist PD 102807 (250 nM). Data are presented as percentage of potentiation (mean ± SEM) of NMDAR current (∗, P < 0.05; n = 5 for each condition).
The Carbachol-Induced Potentiation of NMDA Currents in CA1 Pyramidal Cells Is Blocked by m1-Toxin.
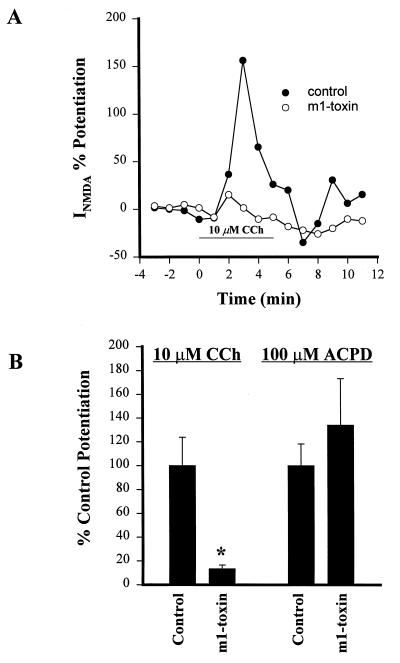
While the studies described above suggest a role for m1 in mediating CCh-induced potentiation of INMDA, the degree of selectivity of the compounds used in these studies does not allow complete confidence in this conclusion. Thus, to further test the hypothesis that the m1 mAChR mediates the muscarinic-induced potentiation of INMDA, we employed the highly specific m1-toxin (25–28). m1-Toxin is an irreversible antagonist of the m1 mAChR, but has no effects on m2, m3, or m5 mAChR subtypes. High concentrations of m1-toxin can inhibit m4. However, the potency of this toxin at m4 is approximately 100-fold lower than its potency at m1. Furthermore, in contrast to its effects at m1, binding of m1-toxin to m4 is fully reversible. Because of this, effects of m1-toxin on mAChR-mediated responses that persist after thorough washing of the toxin from the tissue can be attributed definitively to m1. We incubated hippocampal slices in 1 unit/ml of m1-toxin for at least 1 hr. This is a minimal concentration of m1-toxin needed for inhibition of m1 and is below the concentration needed to inhibit binding to m4. After the initial incubation, slices were washed in the recording chamber for at least 30 min. These conditions were designed to allow complete block of m1 and eliminate any possibility of binding to m4 (27). Control slices were treated identically, but without m1-toxin. As shown in Fig. 3, this treatment inhibited the CCh-induced potentiation of INMDA in cells obtained from m1-toxin-treated slices (control, 193 ± 46% potentiation, n = 5; m1-toxin, 26 ± 6% potentiation, n = 6).
Figure 3.
m1-Toxin specifically inhibits the CCh-induced potentiation of NMDA-receptor currents. (A) Time course of CCh-induced potentiation of NMDAR current in a representative cell pretreated with m1-toxin (○) and a control cell treated in the same fashion but without m1-toxin (•). (B) Mean (±SEM) data demonstrating that m1-toxin specifically blocks the potentiating effect of CCh but has no effect on the response to 1S,3R-ACPD. (∗, P < 0.05; n = 4–5 for each condition).
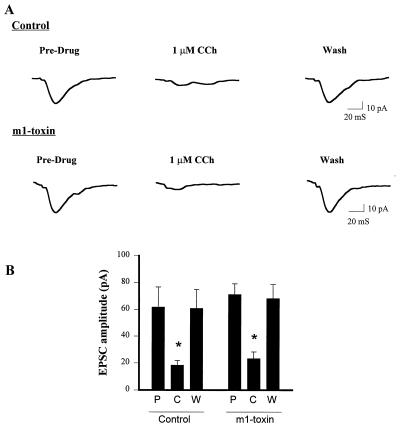
To ensure that m1-toxin did not exert a nonselective effect on potentiation of INMDA, we determined the effect of m1-toxin treatment on potentiation of INMDA by the metabotropic glutamate receptor agonist 1S,3R-ACPD (42, 43). m1-Toxin produced no significant effect on 1S,3R-ACPD-induced potentiation of INMDA (control, 59 ± 12% potentiation, n = 4; m1-toxin, 79 ± 26% potentiation, n = 4) (Fig. 4). To ensure that m1-toxin did not exert nonselective actions on multiple mAChR-mediated responses, we also tested for effects of m1-toxin treatment on CCh-induced inhibition of evoked excitatory postsynaptic currents (EPSCs) at the Schaffer collateral–CA1 synapse. The muscarinic receptor that mediates this effect is unknown; however, it is hypothesized to be a receptor presynaptically localized on the Schaffer collateral terminals that inhibits glutamate release (44). The m1 muscarinic receptor is not highly localized presynaptically (21), and pharmacological characterization of muscarinic inhibition of glutamate release in the hippocampus suggests that either m2 or m4 mediates this effect (45, 46). Thus, it is highly unlikely that this effect is mediated by the m1 receptor. m1-Toxin treatment produced no change in the effect of CCh on evoked EPSCs at this synapse (control inhibition, 70.3 ± 5.7%, n = 5; m1-toxin inhibition, 67.4 ± 7.1%, n = 5) (Fig. 4). Taken together, these data demonstrate that the m1-toxin is selectively inhibiting the CCh-induced potentiation of INMDA and provide strong evidence that this effect is mediated by m1.
Figure 4.
Pretreatment with m1-toxin does not inhibit the CCh-induced reduction of evoked EPSCs. (A) Single traces demonstrating that 1 μM CCh inhibits evoked EPSCs in both control and m1-toxin-treated slices. (B) Mean (±SEM) data showing that CCh produces a significant inhibition of evoked EPSCs in both control and m1-toxin-treated slices (∗, P < 0.05; n = 4 for each condition). P, pre-drug; C, 1 μM CCh; W, washout.
The m1-Muscarinic Receptor Is Colocalized with the NMDA Receptor.
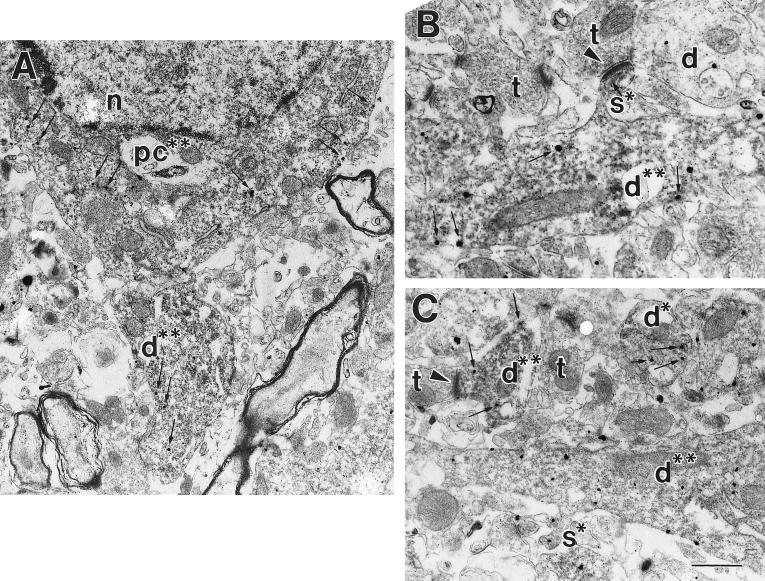
Another prediction of the hypothesis that mAChR-induced potentiation of INMDA is mediated by m1 is that m1 and NMDA receptor subunits should be colocalized in close proximity at glutamatergic synapses. To test for this, we employed double-labeling immunocytochemistry with antibodies that specifically react with m1 and the NR1a subunit of the NMDAR. Immunogold labeling was used for detection of m1 immunoreactivity whereas DAB was used for detection of NR1. Analysis at the electron-microscopy level revealed immunoreactivity for both m1 and NR1 in CA1 pyramidal cell bodies, proximal dendrites, and distal dendrites (Fig. 5). The gold particles that were labeling the m1 protein were seen along the cell membrane of pyramidal cell bodies and proximal and distal dendrites. The DAB reaction product, a diffuse electron-dense precipitant, labeling NR1, was seen inside the same profiles (Fig. 5). Virtually all of the profiles that contained immunogold particles (m1) also contained DAB immunoreactivity (NMDAR), suggesting that all sites containing m1 also contain NMDARs. However, definitive quantification of the extent of colocalization of these two proteins was precluded by the poor penetration of the gold secondary antibodies. Control experiments in which one of the primary antibodies (NR1 or m1) was omitted verified that no crossreactivity was seen. For example, no DAB reaction product was seen when the NR1a antibody was omitted, and the distribution of gold particles labeling m1 was identical to that seen in the double-labeled condition. These anatomical experiments indicate that pyramidal cells express both the m1 receptor and the NMDARs and transport them to dendrites, where they coexist in the same postsynaptic structures.
Figure 5.
The m1-muscarinic receptor colocalizes with the NMDA NR1 subunit. Double-labeling electron microscopic immunocytochemistry was used to identify immunoreactivity for the m1 mAChR (visualized with silver-enhanced immunogold, electron-dense round particles) and the NR1 subunit of the NMDAR protein (visualized with DAB; a diffuse floccular reaction product). (A) A pyramidal cell soma (pc**) that contains immunoreactivity for m1 and NR1. Note the presence of DAB (dark diffuse-reaction product filling cytoplasm, and immunogold particles present in the cytoplasm and lining the membrane highlighted with small arrows in A–C). Also pictured is a large proximal dendrite (d**) double-labeled with both m1 and NMDAR immunoreactivity. n, nucleus. (B) A large proximal dendrite (d**) that contains immunoreactivity for both the m1 and NMDARs. A dendritic spine (s*) containing NMDA immunoreactivity is seen extending off the proximal dendrite that is receiving an asymmetric (excitatory) synapse (arrowhead) from an unlabeled synaptic terminal (t). An unlabeled dendrite (d) is also pictured. (C) Several proximal and distal dendrites that are double-labeled for m1 and NMDAR proteins (d**) are shown. The two dendrites at the top of the panel are smaller distal dendrites, while the one at the bottom is a larger proximal dendrite. The distal dendrite on the top left can be seen receiving an asymmetric synapse (arrowhead) from an unlabeled terminal (t). Additionally, a dendritic spine (s*) containing immunoreactivity for the m1 receptor is shown extending off of the proximal dendrite. [Bar = 1.2 μm (A); 465 nm (B); 440 nm (C).]
DISCUSSION
The present studies provide converging lines of evidence that suggest that activation of the m1 mAChR subtype results in potentiation of INMDA in hippocampal CA1 pyramidal cells. The pharmacological profile of mAChR-induced potentiation of INMDA is consistent with mediation by m1, and this response is irreversibly blocked by the highly specific m1-toxin. Furthermore, double-labeling immunocytochemistry reveals that the m1 and NMDARs colocalize at putative glutamatergic synapses.
The mechanism by which m1 mAChR activation potentiates INMDA currently is unknown. Receptors of the m1 subtype are known to couple to activation of phosphoinositide hydrolysis (19), and Markram and Segal (15) have suggested that the potentiating effect of CCh on INMDA is mediated by activation of this signaling pathway. These findings, along with the demonstration that protein kinase C (PKC) can potentiate INMDA in some systems (47–49), suggest that PKC could underlie this phenomenon. In fact, metabotropic glutamate receptor agonists have been shown to potentiate NMDAR current in hippocampal neurons in a PKC-dependent manner (42, 43). However, pharmacological blockade of PKC does not inhibit the muscarinic-induced potentiation of INMDA in CA1 pyramidal cells (15, 16). Furthermore, direct activation of PKC in these cells inhibits, rather than potentiates, NMDA-evoked currents (50).
Another potential mechanism by which m1 activation could potentiate INMDA is through the activation of tyrosine kinase cascades. Recent studies reveal that the src family of tyrosine kinases can potentiate INMDA and that this may be mediated by phosphorylation of tyrosine in the C-terminal domain of the NR2A subunit (51–54). Interestingly, activation of muscarinic receptors can activate tyrosine kinases in hippocampal slices (60, 61), suggesting that m1-induced activation of tyrosine kinase could mediate the m1-induced potentiation of INMDA. However, future studies will be needed to rigorously test this hypothesis.
At present, the physiological role of m1 mAChR potentiation of NMDAR current and the physiological circumstances under which this response might occur are not entirely clear. The stratum radiatum of area CA1 receives a prominent, yet diffuse innervation of ChAT-positive terminals that rarely make defined synaptic junctions (55). This finding has led some researchers to postulate that modulation by ACh occurs through a diffuse transmission that relies on nonsynaptic changes in ambient ACh levels (55, 56). While it is not known whether ambient levels of ACh are sufficient to effect the Schaffer collateral–CA1 glutamatergic terminals, several studies have shown that stimulation of cholinergic afferents evokes marked increases in CA1 pyramidal neuron excitability through activation of mAChRs (57–59). A definitive statement as to the physiological role of this phenomenon must await further study.
One of the more interesting aspects of the current findings is the possible implications of these studies for development of novel strategies for the treatment of memory loss and other disorders involving hippocampal circuits. Unfortunately, the clinical use of cholinesterase inhibitors such as tacrine is limited because of poor efficacy or side effects associated with their nonselective mechanism of action. Inhibition of acetylcholinesterase results in activation of virtually all ACh receptors, both in the central nervous system and in the periphery. However, m1 plays a relatively minor role in mediating the peripheral effects of ACh and is responsible for only a portion of the effects of ACh in the brain. Thus, development of potent and selective m1 agonists might provide agents with fewer side effects and greater efficacy than currently available compounds.
There are also implications of these studies for treatment of other neurological and psychiatric disorders. For instance, NMDAR-mediated excitotoxicity has been implicated in a number of pathologies, including stroke, head injury, and certain neurodegenerative disorders. It is possible that antagonists of m1 or other receptors involved in potentiation of NMDAR function could be used for reducing NMDAR-mediated excitotoxicity in some conditions. Potentially more promising are the possible implications of these findings in the development of novel treatments for psychiatric disorders such as schizophrenia. Recent clinical and basic studies have led to wide acceptance of the hypothesis that a reduction in transmission at glutamatergic synapses and, especially, reduced NMDAR function underlie some of the pathological changes associated with schizophrenia (see refs. 62 and 63 for reviews). Based on these findings, there has been a major focus on the development of compounds that potentiate NMDAR function in hippocampal and cortical circuits that may alleviate the symptoms of schizophrenia without causing serious side effects. Interestingly, clinical trials of the mAChR agonist xanomeline in Alzheimer’s disease patients revealed a striking reduction in psychotic behaviors such as hallucinations and delusions (64). These findings, coupled with the present data, suggest that the m1 mAChR could provide an excellent target for the development of antipsychotic agents that enhance the NMDA component of glutamatergic synaptic transmission.
The present study shows that the m1 mAChR mediates the muscarinic-induced potentiation of NMDAR currents in hippocampal CA1 pyramidal cells. However, this is not the only mechanism by which ACh could potentiate glutamatergic transmission in the central nervous system. For example, in cortical pyramidal neurons, the m2 mAChR is localized in dendritic spines in close proximity to glutamatergic terminals, raising the possibility that m2 could mediate a potentiation of NMDAR currents at these synapses (65). Furthermore, recent studies have shown that activation of presynaptic nicotinic ACh receptors can enhance glutamatergic transmission by directly increasing presynaptic intracellular calcium concentrations (66). Therefore, ACh could potentially act on presynaptic nicotinic receptors to increase release while potentiating the effects of glutamate postsynaptically by modulation of the NMDAR.
Acknowledgments
We thank Hong Yi for expert technical assistance. This work was supported by National Institutes of Health Grants NS10148 (M.J.M.), NS30454 (A.I.L.), NS31373, NS34876, NS36755 (P.J.C.), and a grant from the Council for Tobacco Research (P.J.C.).
ABBREVIATIONS
- NMDA
N-methyl-d-aspartate
- ACh
acetylcholine
- CCh
carbachol
- mAChR
muscarinic ACh receptor
- ACSF
artificial cerebrospinal fluid
- DAB
diaminobenzidine
- EPSC
excitatory postsynaptic current
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Brown T H, Zador A M. In: The Synaptic Organization of the Brain. Shepherd G M, editor. New York: Oxford Univ. Press; 1990. pp. 346–388. [Google Scholar]
- 2.Drachman D A, Leavitt J L. Arch Neurol. 1974;30:113–121. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- 3.Bartus R T, Dean D L I, Beer B, Lippa A S. Science. 1982;217:783–790. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 4.Dekker A J A M, Connor D J, Thal L J. Neurosci Biobehav Rev. 1991;15:299–317. doi: 10.1016/s0149-7634(05)80008-9. [DOI] [PubMed] [Google Scholar]
- 5.Fibiger H C. Trends Neurosci. 1991;14:220–223. doi: 10.1016/0166-2236(91)90117-d. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson O G, Leanza G, Rosenblad C, Lappi D A, Wiley R G, Bjorklund A. NeuroReport. 1992;3:1005–1008. doi: 10.1097/00001756-199211000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Callahan M J, Kinsora J J, Harbaugh R E, Reeder T M, Davis R E. Neurobiol Aging. 1993;14:147–151. doi: 10.1016/0197-4580(93)90090-x. [DOI] [PubMed] [Google Scholar]
- 8.Bowen D M, Smith C B, White P, Davison A N. Brain Res. 1976;99:459–496. doi: 10.1093/brain/99.3.459. [DOI] [PubMed] [Google Scholar]
- 9.Davies P, Maloney A J F. Nature (London) 1976;288:279–280. [Google Scholar]
- 10.Whitehouse P J, Price D L, Struble R G, Clark A W, Coyle J T, Delong M R. Science. 1982;215:1237–1239. [Google Scholar]
- 11.Arendt T, Bigl V, Arendt A, Tennstedt A. Acta Neuropathol. 1983;61:101–108. doi: 10.1007/BF00697388. [DOI] [PubMed] [Google Scholar]
- 12.Reinikainen K J, Soininen H, Rienkkinen P J. J Neurosci Res. 1990;27:576–586. doi: 10.1002/jnr.490270419. [DOI] [PubMed] [Google Scholar]
- 13.Markram H, Segal M. J Physiol. 1990;427:381–393. doi: 10.1113/jphysiol.1990.sp018177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markram H, Segal M. Neurosci Lett. 1990;113:62–65. doi: 10.1016/0304-3940(90)90495-u. [DOI] [PubMed] [Google Scholar]
- 15.Markram H, Segal M. J Physiol. 1992;447:513–533. doi: 10.1113/jphysiol.1992.sp019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvey J, Collingridge G L. Br J Pharmacol. 1993;109:1085–1090. doi: 10.1111/j.1476-5381.1993.tb13733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McBain C J, Mayer M L. Physiol Rev. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- 18.Peralta E G, Ashkenazi A, Winslow J W, Smith D H, Ramachandran J, Capon D J. EMBO J. 1987;6:3923–3929. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caulfield M P. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 20.Hulme E C, Birdsall N J M, Buckley N J. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- 21.Levey A I, Edmunds S M, Koliatsos V, Wiley R G, Heilman C J. J Neurosci. 1995;15:4077–4092. doi: 10.1523/JNEUROSCI.15-05-04077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey A I, Kitt C A, Simonds W F, Price D L, Brann M R. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mrzljak L, Levey A I, Goldman-Rakic P S. Proc Natl Acad Sci USA. 1993;90:5194–5198. doi: 10.1073/pnas.90.11.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouse S T, Gilmor M L, Levey A I. Neuroscience. 1998;86:221–232. doi: 10.1016/s0306-4522(97)00681-7. [DOI] [PubMed] [Google Scholar]
- 25.Max S I, Liang J-S, Potter L T. J Neurosci. 1993;13:4293–4300. doi: 10.1523/JNEUROSCI.13-10-04293.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Max S I, Liang J-S, Potter L T. Mol Pharmacol. 1993;44:1171–1175. [PubMed] [Google Scholar]
- 27.Max S I, Liang J-S, Valentine H H, Potter L T. J Pharmacol Exp Ther. 1993;267:480–485. [PubMed] [Google Scholar]
- 28.Jerusalinsky D, Harvey A L. Trends Pharmacol Sci. 1994;15:424–430. doi: 10.1016/0165-6147(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 29.Aubert I, Cécyre D, Gauthier S, Quirion R. J Comp Neurol. 1996;369:31–55. doi: 10.1002/(SICI)1096-9861(19960520)369:1<31::AID-CNE3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 30.Monyer H, Burnashev N, Laurie D J, Sakmann B, Seeburg P H. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 31.Gereau R W, Conn P J. J Neurosci. 1995;15:6879–6889. doi: 10.1523/JNEUROSCI.15-10-06879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegel S J, Brose N, Janssen W G, Gasic G P, Jahn R, Heinemann S F, Morrison J H. Proc Natl Acad Sci USA. 1994;91:564–568. doi: 10.1073/pnas.91.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brose N, Huntley G W, Stern-Bach Y, Sharma G, Morrison J H, Heinemann S F. J Biol Chem. 1994;269:16780–16784. [PubMed] [Google Scholar]
- 34.Hersch S M, Gutekunst C A, Reese H D, Heilman C J, Levey A I. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burry R, Vandre D, Hayes D. J Histochem Cytochem. 1992;40:1849–1856. doi: 10.1177/40.12.1453003. [DOI] [PubMed] [Google Scholar]
- 36.Janowsky A, Labarca R, Paul S M. Life Sci. 1984;35:1935–1961. doi: 10.1016/0024-3205(84)90476-4. [DOI] [PubMed] [Google Scholar]
- 37.Fisher S K, Bartus R T. J Neurochem. 1985;45:1085–1095. doi: 10.1111/j.1471-4159.1985.tb05527.x. [DOI] [PubMed] [Google Scholar]
- 38.Fisher S K, Snider R M. Mol Pharm. 1987;32:81–90. [PubMed] [Google Scholar]
- 39.Dorje F, Wess J, Lambrecht G, Tacke R, Mutschler E, Brann M R. J Pharm Exp Ther. 1991;256:727–733. [PubMed] [Google Scholar]
- 40.Augelli-Szafran C E, Moreland D W, Nelson C, Penvose-Yi J R, Schwarz R D, Jaen J C. Soc Neurosci Abstr. 1996;26:500.15. [Google Scholar]
- 41.Schwarz R D, Nelson C B, Augelli-Szafran C E, Penvose J R, Jaen J C, Wiley J, Frey K A. Soc Neurosci Abstr. 1996;26:500.16. [Google Scholar]
- 42.Aniksztejn L, Bregestovski P, Ben-Ari Y. Eur J Pharmacol. 1991;205:327–328. doi: 10.1016/0014-2999(91)90921-c. [DOI] [PubMed] [Google Scholar]
- 43.Aniksztejn L, Otani S, Ben-Ari Y. Eur J Neurosci. 1992;4:500–505. doi: 10.1111/j.1460-9568.1992.tb00900.x. [DOI] [PubMed] [Google Scholar]
- 44.Valentino R, Dingledine R. J Neurosci. 1981;1:784–792. doi: 10.1523/JNEUROSCI.01-07-00784.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marchi M, Bocchieri P, Garbarino L, Raiteri M. Neurosci Lett. 1989;96:229–234. doi: 10.1016/0304-3940(89)90063-3. [DOI] [PubMed] [Google Scholar]
- 46.Raiteri M, Marchi M, Costi A, Volpe G. Eur J Pharmacol. 1990;177:181–187. doi: 10.1016/0014-2999(90)90268-b. [DOI] [PubMed] [Google Scholar]
- 47.Chen L, Huang L-Y M. Neuron. 1991;7:319–326. doi: 10.1016/0896-6273(91)90270-a. [DOI] [PubMed] [Google Scholar]
- 48.Chen L, Huang L-Y M. Nature (London) 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- 49.Blank T, Zwart R, Nijholt T, Spiess J. J Neurosci Res. 1996;45:153–160. doi: 10.1002/(SICI)1097-4547(19960715)45:2<153::AID-JNR7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 50.Markram H, Segal M. J Physiol. 1992;457:491–501. doi: 10.1113/jphysiol.1992.sp019389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y T, Salter M W. Nature (London) 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- 52.Köhr G, Seeburg P H. J Physiol. 1996;492:445–452. doi: 10.1113/jphysiol.1996.sp021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu X M, Askalan R, Keil G J, Salter M W. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- 54.Zheng F, Gingrich M B, Traynelis S F, Conn P J. Nat Neurosci. 1998;1:185–191. doi: 10.1038/634. [DOI] [PubMed] [Google Scholar]
- 55.Umbriaco D, Garcia S, Beaulieu C, Descarries L. Hippocampus. 1995;5:605–620. doi: 10.1002/hipo.450050611. [DOI] [PubMed] [Google Scholar]
- 56.Descarries L, Gisiger V, Steriade M. Prog Neurobiol. 1997;53:603–625. doi: 10.1016/s0301-0082(97)00050-6. [DOI] [PubMed] [Google Scholar]
- 57.Krnjević K, Ropert N. Neuroscience. 1982;7:165–2183. doi: 10.1016/0306-4522(82)90128-2. [DOI] [PubMed] [Google Scholar]
- 58.Cole A E, Nicoll R A. J Physiol. 1984;352:173–188. doi: 10.1113/jphysiol.1984.sp015285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morton R A, Davies C H. J Physiol. 1997;502.1:75–90. doi: 10.1111/j.1469-7793.1997.075bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stratton K R, Worley P F, Huganir R L, Baraban J M. Proc Natl Acad Sci USA. 1989;86:2498–2501. doi: 10.1073/pnas.86.7.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siciliano J C, Gelman M, Girault J-A. J Neurochem. 1994;62:950–959. doi: 10.1046/j.1471-4159.1994.62030950.x. [DOI] [PubMed] [Google Scholar]
- 62.Olney J W, Farber N B. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 63.Hirsch S R, Das I, Garey L J, de Belleroche J. Pharmacol Biochem Behav. 1997;56:797–802. doi: 10.1016/s0091-3057(96)00428-5. [DOI] [PubMed] [Google Scholar]
- 64.Bodick N C, Offen W W, Levey A I, Cutler N R, Gauthier S G, Satlin A, Shannon H E, Tollefson G D, Rasmussen K, Bymaster F P, et al. Arch Neurol. 1997;54:465–473. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- 65.Mrzljak L, Levey A I, Belcher S, Goldman-Rakic P S. J Comp Neurol. 1998;390:112–132. [PubMed] [Google Scholar]
- 66.McGehee D S, Heath M J S, Gelber S, Devay P, Role L W. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]