Abstract
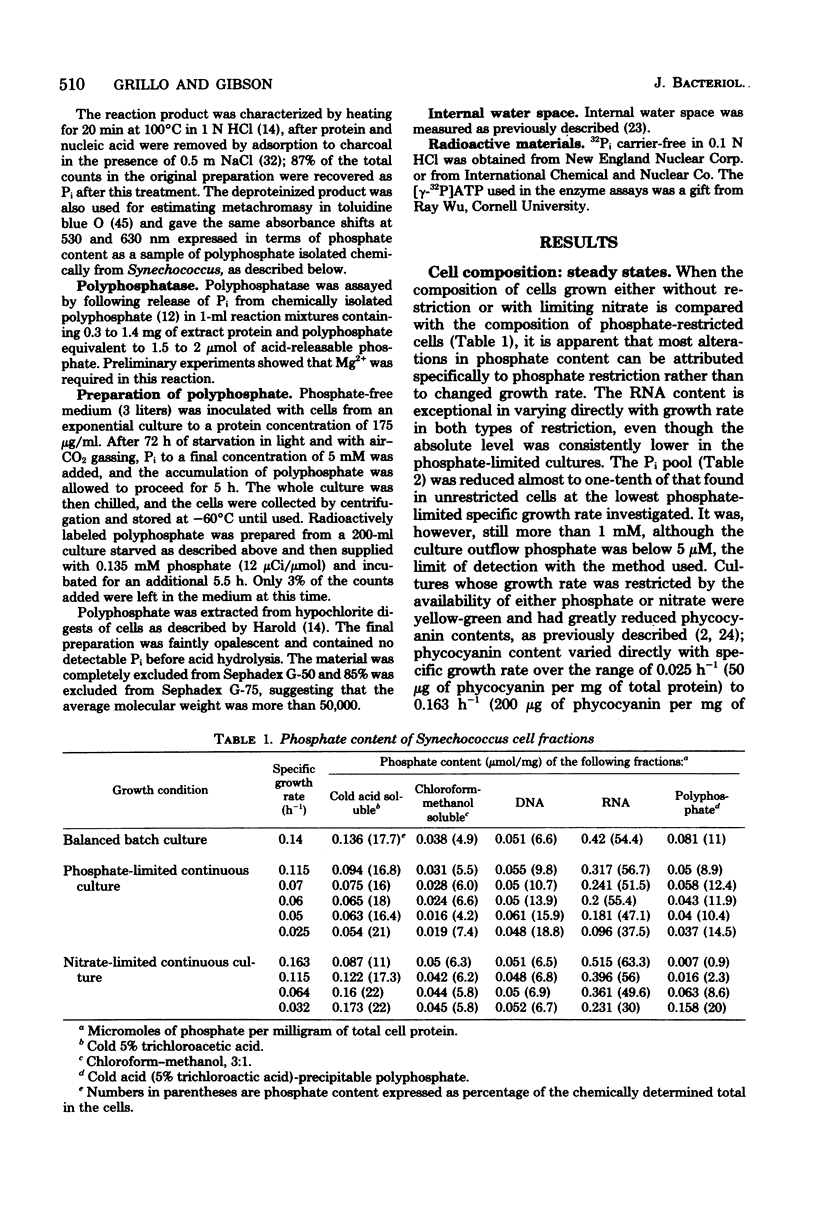
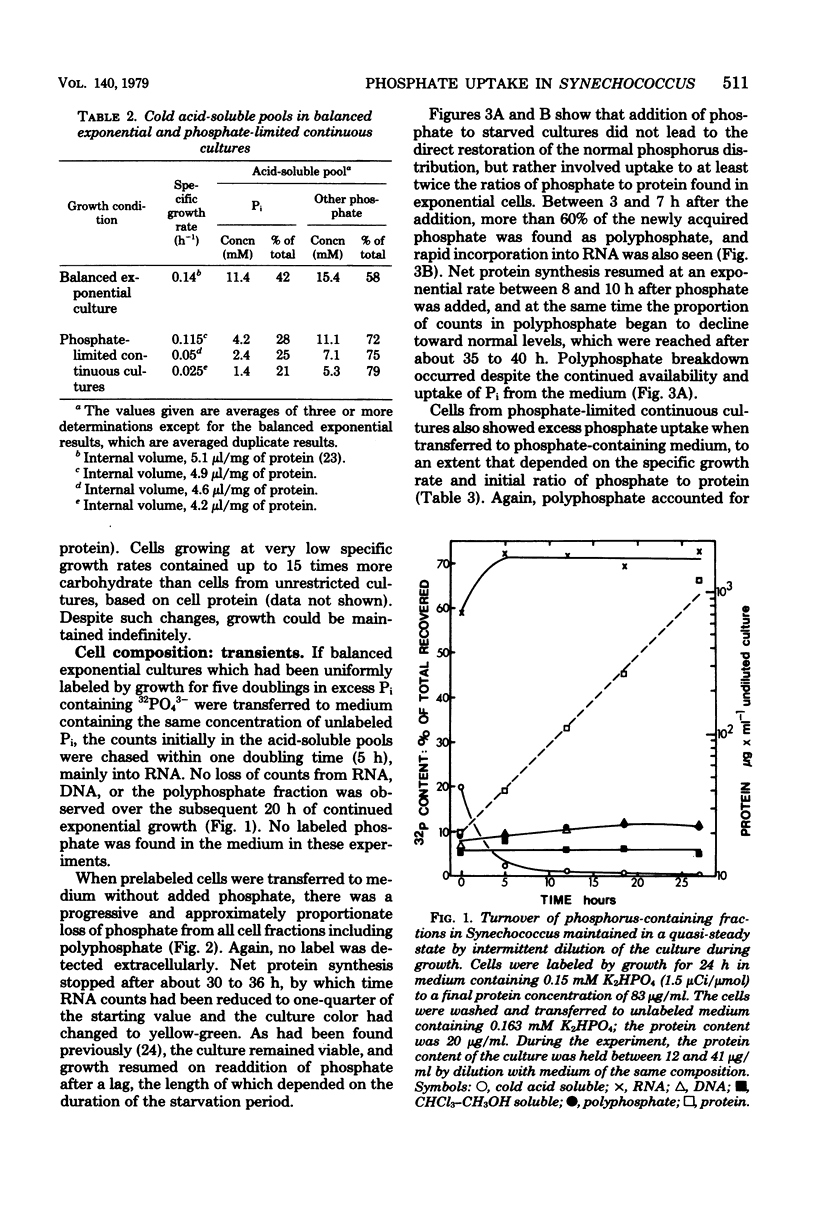
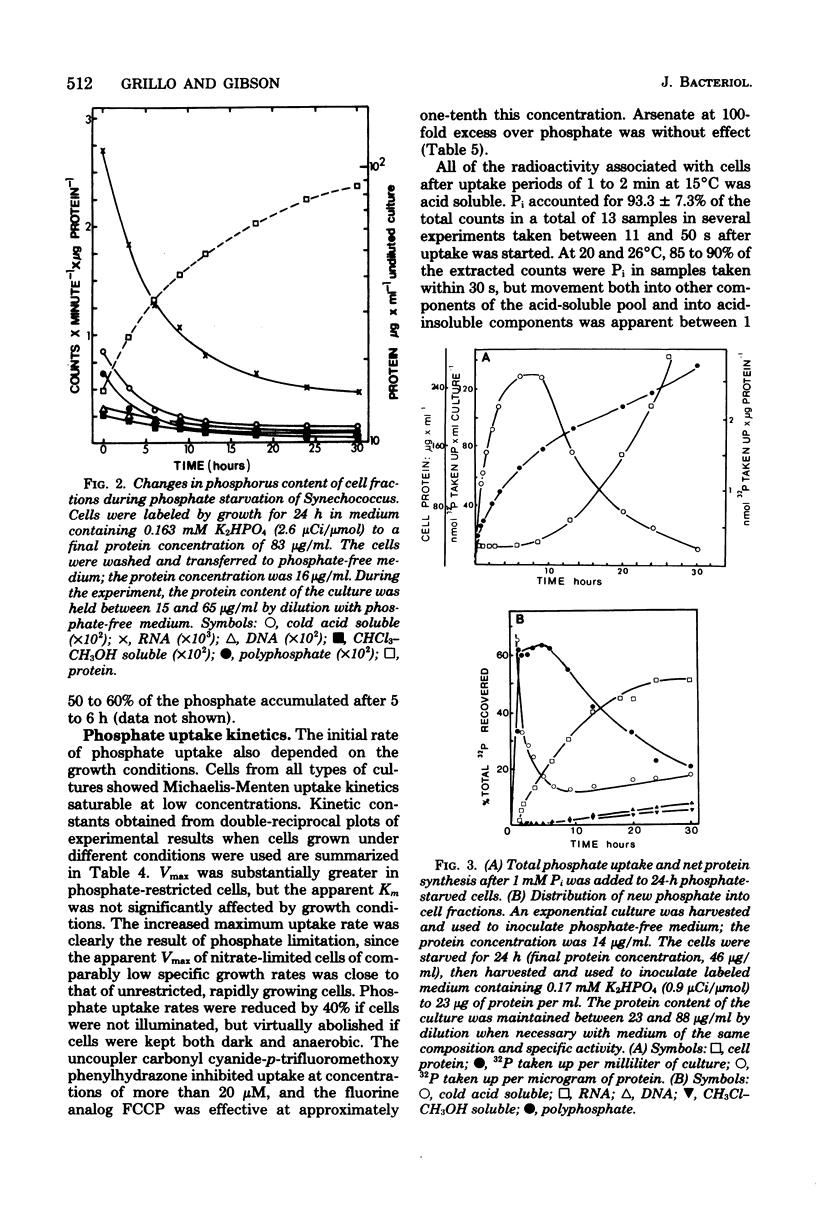
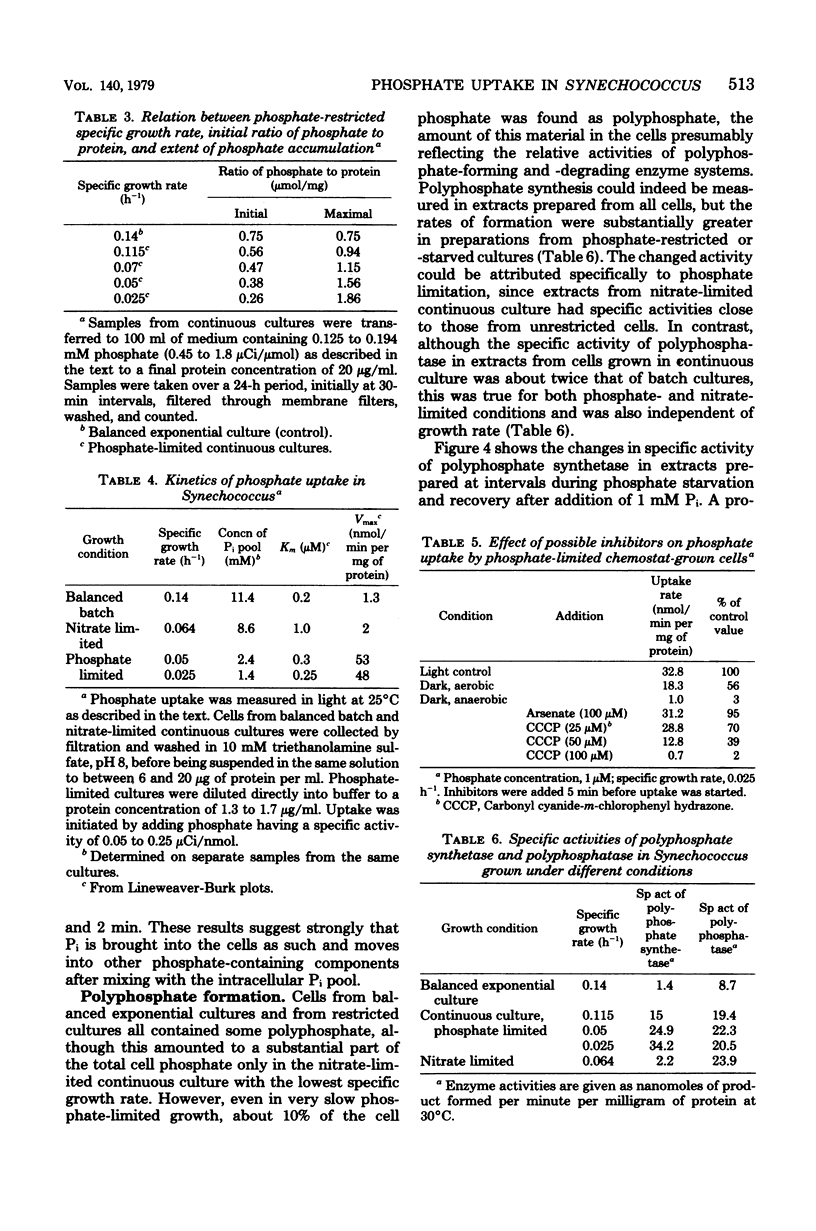
The phosphorus contents of acid-soluble pools, lipid, ribonucleic acid, and acid-insoluble polyphosphate were lowered in Synechococcus in proportion to the reduction in growth rate in phosphate-limited but not in nitrate-limited continuous culture. Phosphorus in these cell fractions was lost proportionately during progressive phosphate starvation of batch cultures. Acid-insoluble polyphosphate was always present in all cultural conditions to about 10% of total cell phosphorus and did not turn over during balanced exponential growth. Extensive polyphosphate formation occurred transiently when phosphate was given to cells which had been phosphate limited. This material was broken down after 8 h even in the presence of excess external orthophosphate, and its phosphorus was transferred into other cell fractions, notably ribonucleic acid. Phosphate uptake kinetics indicated an invariant apparent Km of about 0.5 μM, but Vmax was 40 to 50 times greater in cells from phosphate-limited cultures than in cells from nitrate-limited or balanced batch cultures. Over 90% of the phosphate taken up within the first 30 s at 15°C was recovered as orthophosphate. The uptake process is highly specific, since neither phosphate entry nor growth was affected by a 100-fold excess of arsenate. The activity of polyphosphate synthetase in cell extracts increased at least 20-fold during phosphate starvation or in phosphate-restricted growth, but polyphosphatase activity was little changed by different growth conditions. The findings suggest that derepression of the phosphate transport and polyphosphate-synthesizing systems as well as alkaline phosphatase occurs in phosphate shortage, but that the breakdown of polyphosphate in this organism is regulated by modulation of existing enzyme activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Allen M. M., Smith A. J. Nitrogen chlorosis in blue-green algae. Arch Mikrobiol. 1969;69(2):114–120. doi: 10.1007/BF00409755. [DOI] [PubMed] [Google Scholar]
- Batterton J. C., Van Baalen C. Phosphorus deficiency and phosphate uptake in the blue-green alga Anacystis nidulans. Can J Microbiol. 1968 Apr;14(4):341–348. doi: 10.1139/m68-056. [DOI] [PubMed] [Google Scholar]
- Bone D. H. Relationship between phosphates and alkaline phosphatase of Anabaena flos-aquae in continuous culture. Arch Mikrobiol. 1971;80(2):147–153. doi: 10.1007/BF00411879. [DOI] [PubMed] [Google Scholar]
- DRYER R. L., TAMMES A. R., ROUTH J. I. The determination of phosphorus and phosphatase with N-phenyl-p-phenylenediamine. J Biol Chem. 1957 Mar;225(1):177–183. [PubMed] [Google Scholar]
- Edlin G., Broda P. Physiology and genetics of the "ribonucleic acid control" locus in escherichia coli. Bacteriol Rev. 1968 Sep;32(3):206–226. doi: 10.1128/br.32.3.206-226.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gezelius K. Inorganic polyphosphates and enzymes of polyphosphate metabolism in the cellular slime mold Dictyostelium discoideum. Arch Microbiol. 1974 Jul 22;98(4):311–329. doi: 10.1007/BF00425292. [DOI] [PubMed] [Google Scholar]
- HAROLD F. M. ACCUMULATION OF INORGANIC POLYPHOSPHATE IN AEROBACTER AEROGENES. I. RELATIONSHIP TO GROWTH AND NUCLEIC ACID SYNTHESIS. J Bacteriol. 1963 Aug;86:216–221. doi: 10.1128/jb.86.2.216-221.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAROLD F. M. ENZYMIC AND GENETIC CONTROL OF POLYPHOSPHATE ACCUMULATION IN AEROBACTER AEROGENES. J Gen Microbiol. 1964 Apr;35:81–90. doi: 10.1099/00221287-35-1-81. [DOI] [PubMed] [Google Scholar]
- HAROLD F. M. INORGANIC POLYPHOSPHATE OF HIGH MOLECULAR WEIGHT FROM AEROBACTER AEROGENES. J Bacteriol. 1963 Oct;86:885–887. doi: 10.1128/jb.86.4.885-887.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAROLD F. M., SYLVAN S. ACCUMULATION OF INORGANIC POLYPHOSPHATE IN AEROBACTER AEROGENES. II. ENVIRONMENTAL CONTROL AND THE ROLE OF SULFUR COMPOUNDS. J Bacteriol. 1963 Aug;86:222–231. doi: 10.1128/jb.86.2.222-231.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAROLD R. L., HAROLD F. M. Mutants of Aerobacter aerogenes blocked in the accumulation of inorganic polyphosphate. J Gen Microbiol. 1963 May;31:241–246. doi: 10.1099/00221287-31-2-241. [DOI] [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Inorganic polyphosphates in biology: structure, metabolism, and function. Bacteriol Rev. 1966 Dec;30(4):772–794. doi: 10.1128/br.30.4.772-794.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Spitz E. Accumulation of arsenate, phosphate, and aspartate by Sreptococcus faecalis. J Bacteriol. 1975 Apr;122(1):266–277. doi: 10.1128/jb.122.1.266-277.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihlenfeldt M. J., Gibson J. CO2 fixation and its regulation in Anacystis nidulans (Synechococcus). Arch Microbiol. 1975;102(1):13–21. doi: 10.1007/BF00428339. [DOI] [PubMed] [Google Scholar]
- Ihlenfeldt M. J., Gibson J. Phosphate utilization and alkaline phosphatase activity in Anacystis nidulans (Synechococcus). Arch Microbiol. 1975;102(1):23–28. doi: 10.1007/BF00428340. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., KORNBERG S. R., SIMMS E. S. Metaphosphate synthesis by an enzyme from Escherichia coli. Biochim Biophys Acta. 1956 Apr;20(1):215–227. doi: 10.1016/0006-3002(56)90280-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MUHAMMED A., RODGERS A., HUGHES D. E. Purification and properties of a polymetaphosphatase from Corynebacterium xerosis. J Gen Microbiol. 1959 Jun;20(3):482–495. doi: 10.1099/00221287-20-3-482. [DOI] [PubMed] [Google Scholar]
- Mühlradt P. F. Synthesis of high molecular weight polyphosphate with a partially purified enzyme from Salmonella. J Gen Microbiol. 1971 Sep;68(1):115–122. doi: 10.1099/00221287-68-1-115. [DOI] [PubMed] [Google Scholar]
- Pelroy R. A., Rippka R., Stanier R. Y. Metabolism of glucose by unicellular blue-green algae. Arch Mikrobiol. 1972;87(4):303–322. doi: 10.1007/BF00409131. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Gerdes R. G., Chegwidden K. Two systems for the uptake of phosphate in Escherichia coli. J Bacteriol. 1977 Aug;131(2):505–511. doi: 10.1128/jb.131.2.505-511.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H., Medveczky N., La Nauze J. M. Phosphate transport in Bacillus cereus. Biochim Biophys Acta. 1969 Oct 14;193(1):159–167. doi: 10.1016/0005-2736(69)90069-8. [DOI] [PubMed] [Google Scholar]
- SUGINO Y., MIYOSHI Y. THE SPECIFIC PRECIPITATION OF ORTHOPHOSPHATE AND SOME BIOCHEMICAL APPLICATIONS. J Biol Chem. 1964 Jul;239:2360–2364. [PubMed] [Google Scholar]
- Schneider K., Frischknecht K. Orthophosphate influx and efflux rates of Chlorella fusca measured in a continuous turbidostat culture with 32P under various conditions. Arch Microbiol. 1977 Dec 15;115(3):339–346. doi: 10.1007/BF00446461. [DOI] [PubMed] [Google Scholar]
- Vaillancourt S., Beuchemin-Newhouse N., Cedergren R. J. Polyphosphate-deficient mutants of Anacystis nidulans. Can J Microbiol. 1978 Feb;24(2):112–116. doi: 10.1139/m78-021. [DOI] [PubMed] [Google Scholar]
- Willsky G. R., Bennett R. L., Malamy M. H. Inorganic phosphate transport in Escherichia coli: involvement of two genes which play a role in alkaline phosphatase regulation. J Bacteriol. 1973 Feb;113(2):529–539. doi: 10.1128/jb.113.2.529-539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



