Abstract
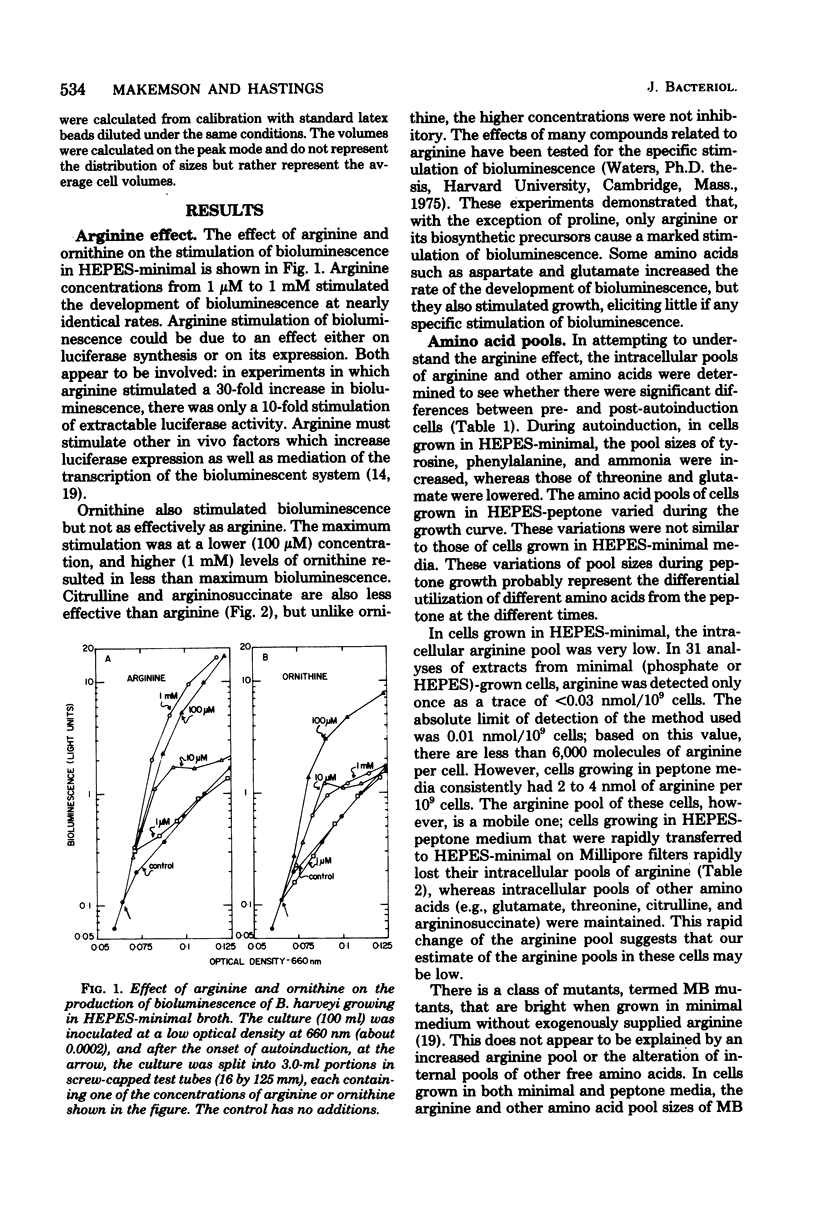
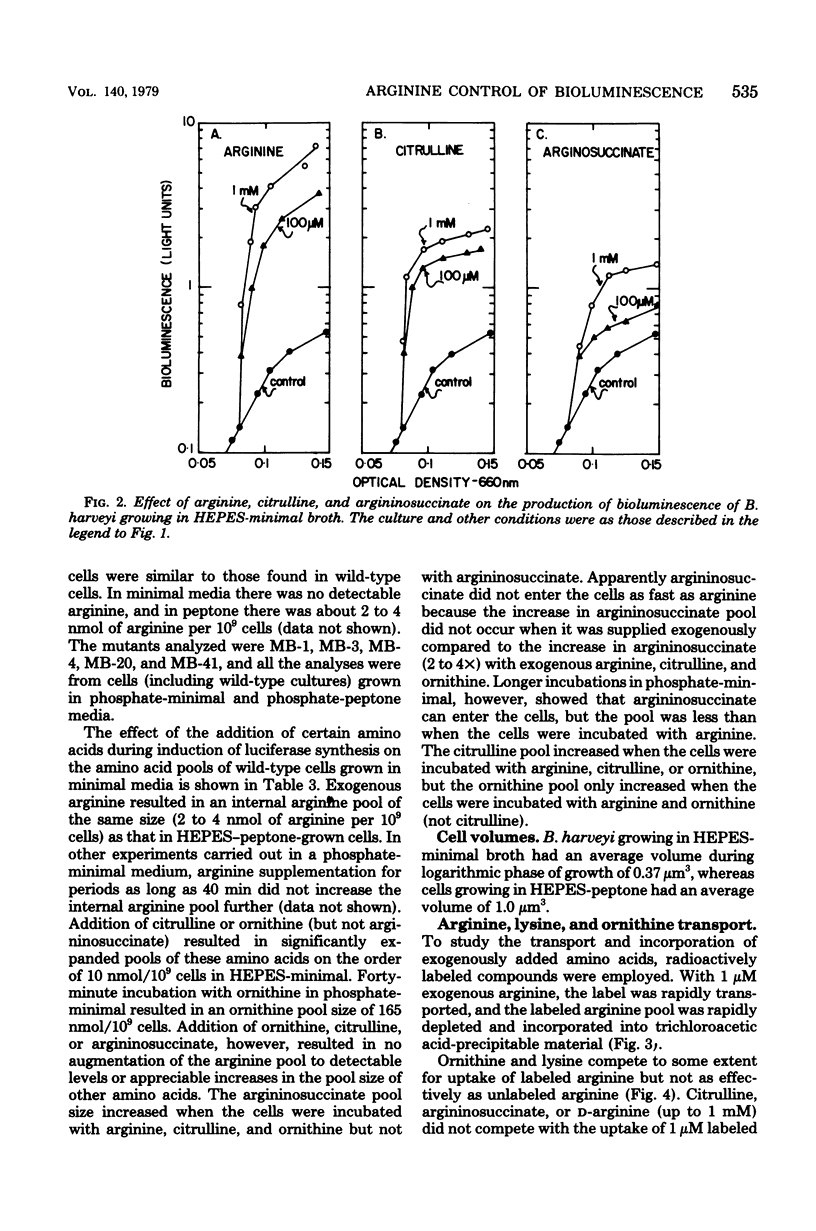
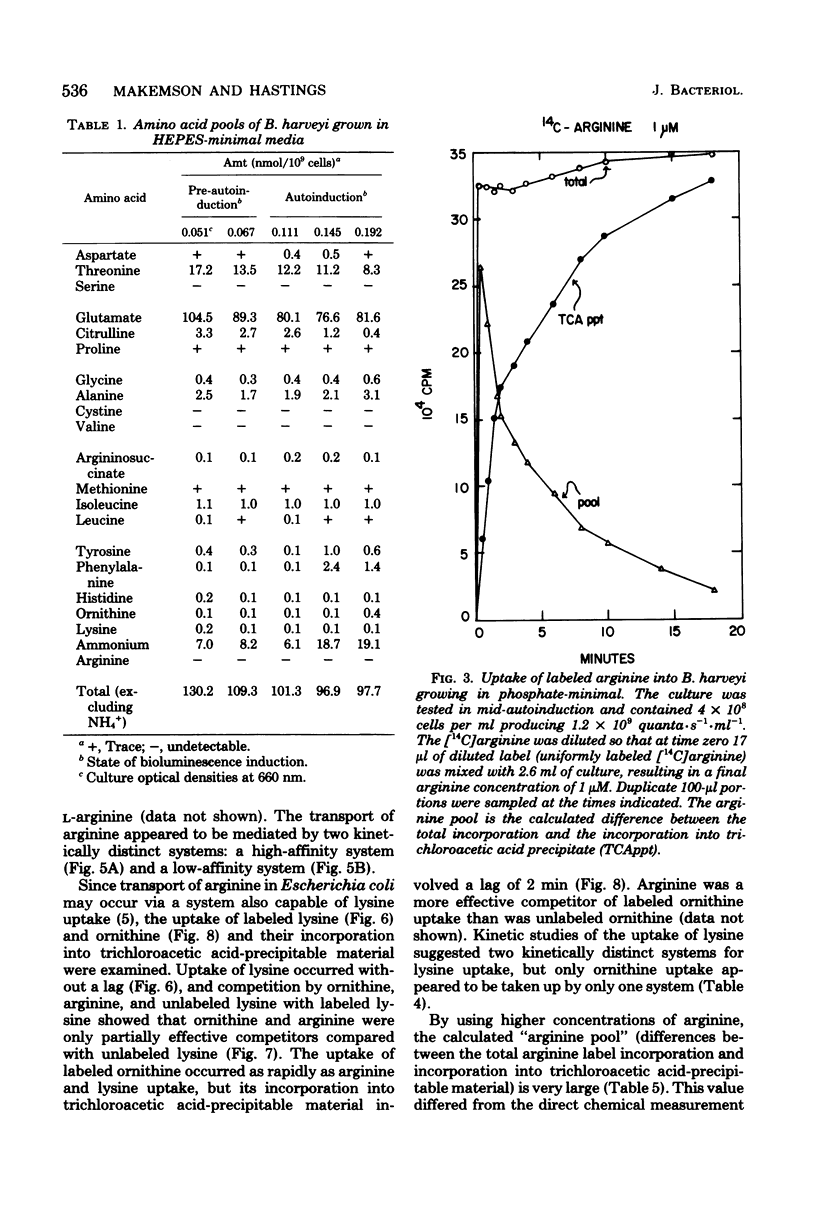
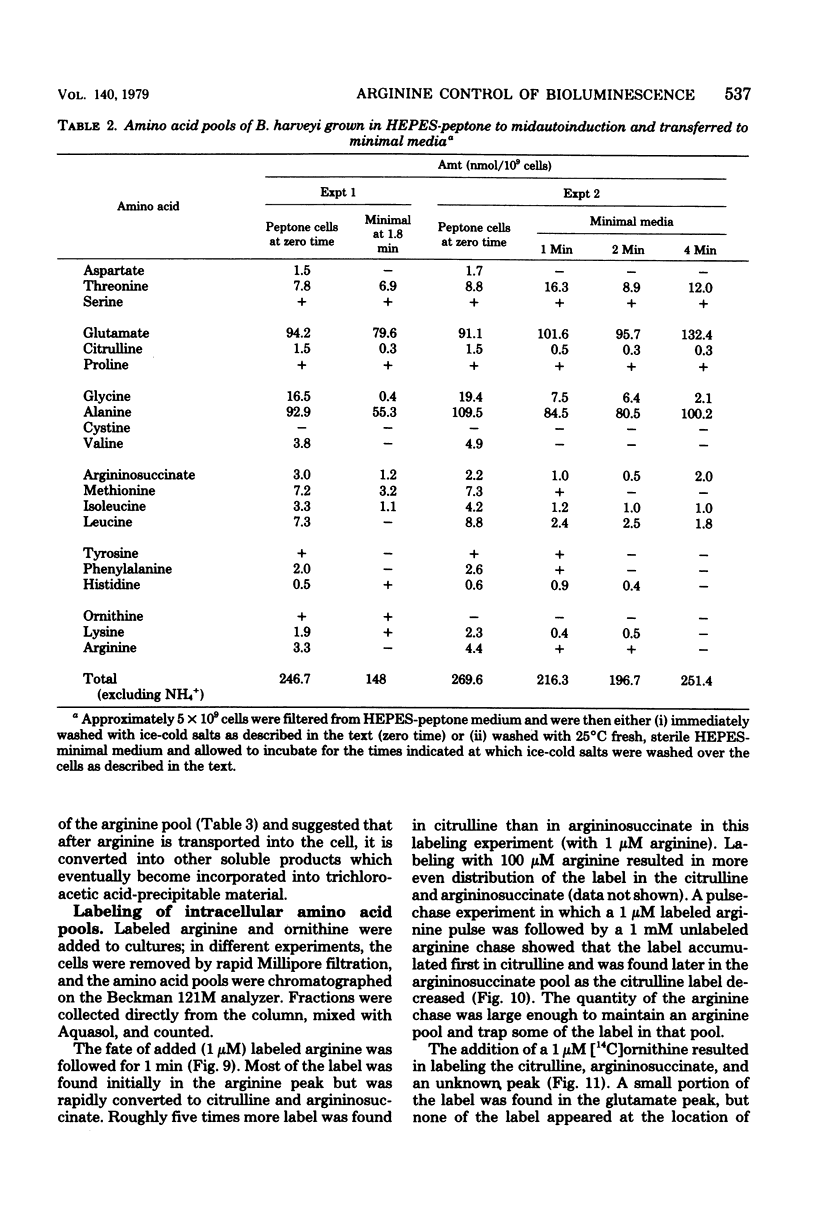
Arginine dramatically stimulates bioluminescence in the marine bacterium Beneckea harveyi growing in minimal media, an effect that is due to increases in both the synthesis and expression of luciferase. To elucidate the mechanism of this phenomenon, studies were made of the transport and metabolism of arginine in B. harveyi. The transport of arginine and lysine involves two kinetically distinct transport systems for the uptake of arginine and lysine. In contrast, ornithine is transported only by a system common to all three amino acids. The internal amino acid pools were measured in mutants that do not show stimulation of bioluminescence by arginine and in wild-type cells that do. In minimal media, the internal arginine pools are undetectably low. Furthermore, exogenously added labeled arginine is rapidly transported and converted to citrulline and argininosuccinate. The results can be accommodated by a model in which the internal arginine is poised at a very low concentration; the stimulatory effect of exogenous arginine on luciferase biosynthesis occurs at the transcriptional level, and the actual mediator can be either arginine or argininyl transfer ribonucleic acid.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P., Baumann L. Biology of the marine enterobacteria: genera Beneckea and Photobacterium. Annu Rev Microbiol. 1977;31:39–61. doi: 10.1146/annurev.mi.31.100177.000351. [DOI] [PubMed] [Google Scholar]
- Celis T. F. Independent regulation of transport and biosynthesis of arginine in Escherichia coli K-12. J Bacteriol. 1977 Jun;130(3):1244–1252. doi: 10.1128/jb.130.3.1244-1252.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis T. F. Properties of an Escherichia coli K-12 mutant defective in the transport of arginine and ornithine. J Bacteriol. 1977 Jun;130(3):1234–1243. doi: 10.1128/jb.130.3.1234-1243.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis T. F., Rosenfeld H. J., Maas W. K. Mutant of Escherichia coli K-12 defective in the transport of basic amino acids. J Bacteriol. 1973 Nov;116(2):619–626. doi: 10.1128/jb.116.2.619-626.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey J. J. Inducible synthesis of bacterial luciferase: specificity and kinetics of induction. J Bacteriol. 1967 Nov;94(5):1638–1647. doi: 10.1128/jb.94.5.1638-1647.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings J. W., Nealson K. H. Bacterial bioluminescence. Annu Rev Microbiol. 1977;31:549–595. doi: 10.1146/annurev.mi.31.100177.003001. [DOI] [PubMed] [Google Scholar]
- Makemson J. C., Hastings J. W. Glutamate functions in osmoregulation in a marine bacterium. Appl Environ Microbiol. 1979 Jul;38(1):178–180. doi: 10.1128/aem.38.1.178-180.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. W., Hastings J. W. A stable, inexpensive, solid-state photomultiplier photometer. Anal Biochem. 1971 Jan;39(1):243–250. doi: 10.1016/0003-2697(71)90481-7. [DOI] [PubMed] [Google Scholar]
- Nealson K. H. Autoinduction of bacterial luciferase. Occurrence, mechanism and significance. Arch Microbiol. 1977 Feb 4;112(1):73–79. doi: 10.1007/BF00446657. [DOI] [PubMed] [Google Scholar]
- Nealson K. H., Eberhard A., Hastings J. W. Catabolite repression of bacterial bioluminescence: functional implications. Proc Natl Acad Sci U S A. 1972 May;69(5):1073–1076. doi: 10.1073/pnas.69.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H., Platt T., Hastings J. W. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970 Oct;104(1):313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raunio R. P., Leppävirta M. The effect of culture age, chloramphenicol and B6 inhibitors on intra- and extracellular keto and amino acids of Escherichia coli B. J Gen Microbiol. 1975 Mar;87(1):141–149. doi: 10.1099/00221287-87-1-141. [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Basic amino acid transport in Escherichia coli: properties of canavanine-resistant mutants. J Bacteriol. 1973 Nov;116(2):627–635. doi: 10.1128/jb.116.2.627-635.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Influence of environment on the content and composition of microbial free amino acid pools. J Gen Microbiol. 1970 Dec;64(2):171–185. doi: 10.1099/00221287-64-2-171. [DOI] [PubMed] [Google Scholar]
- Waters C. A., Hastings J. W. Mutants of luminous bacteria with an altered control of luciferase synthesis. J Bacteriol. 1977 Aug;131(2):519–525. doi: 10.1128/jb.131.2.519-525.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



