Abstract
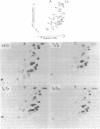
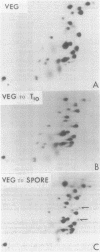
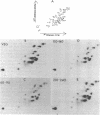
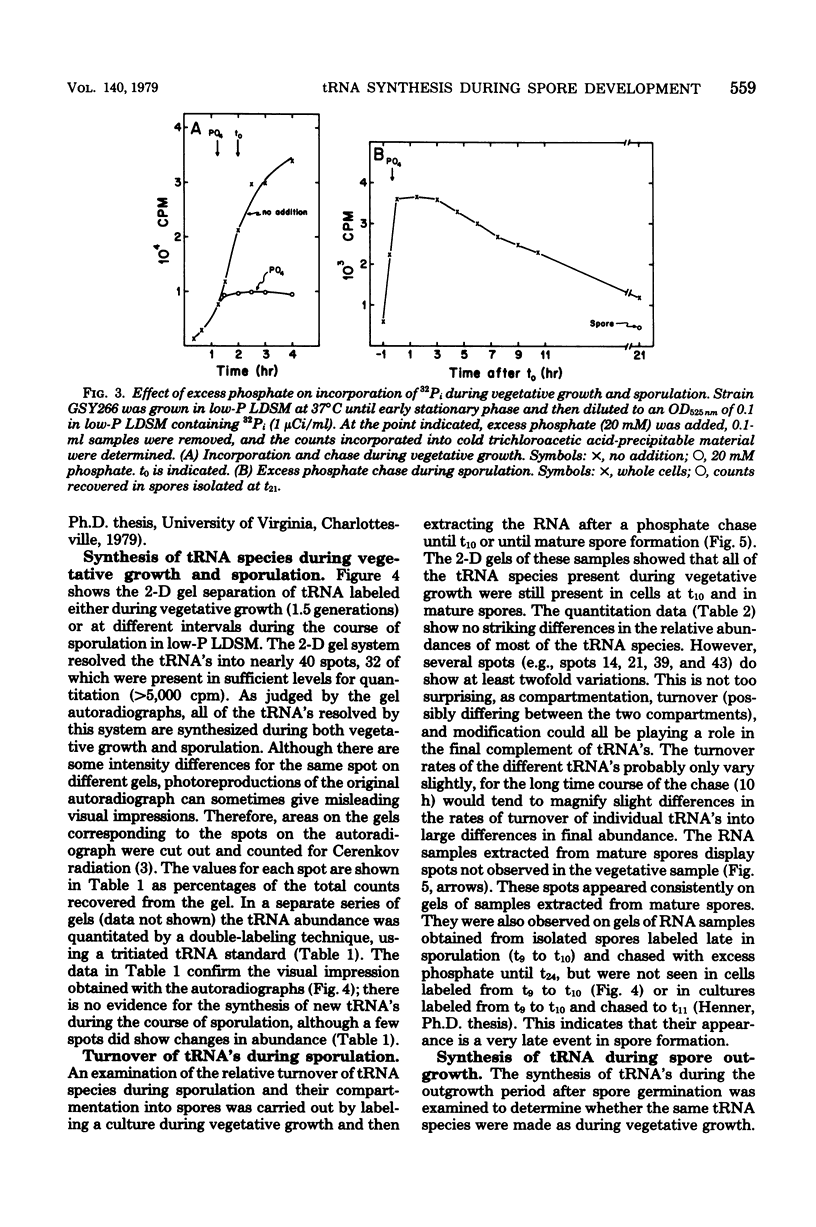
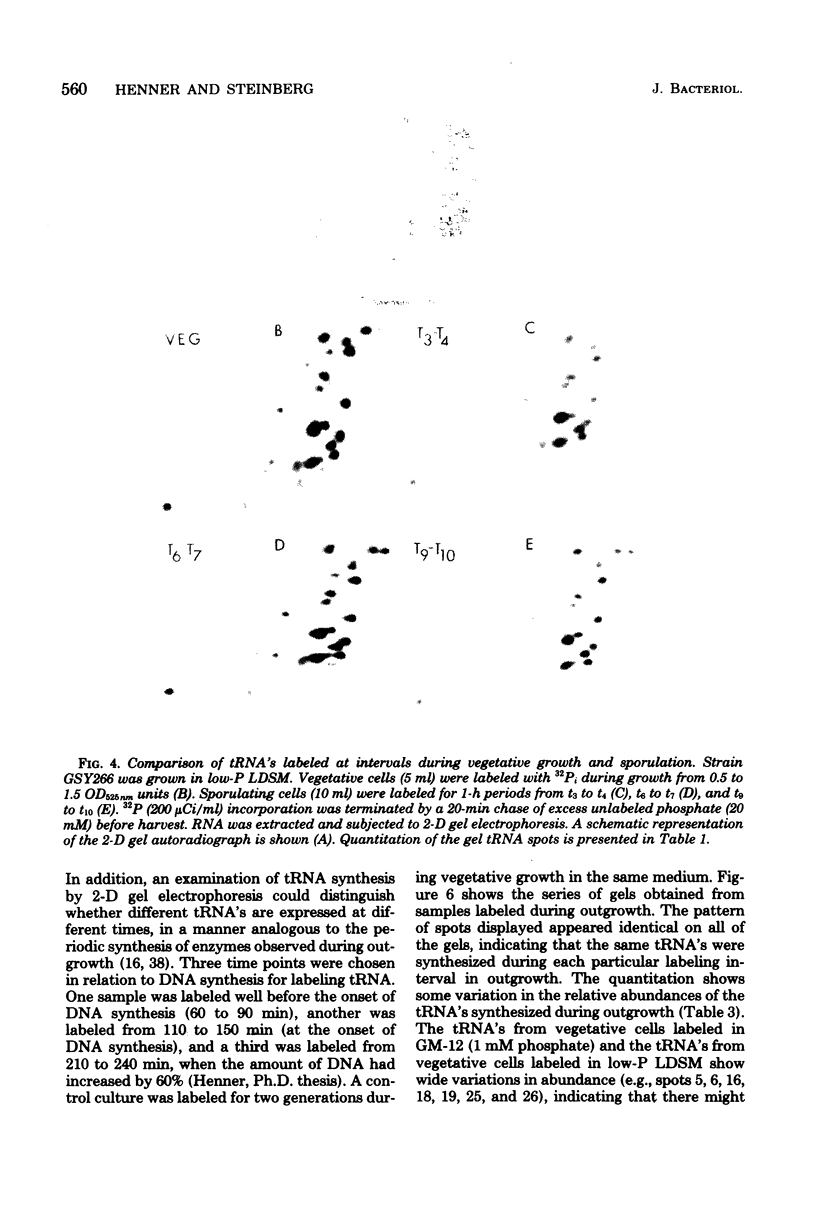
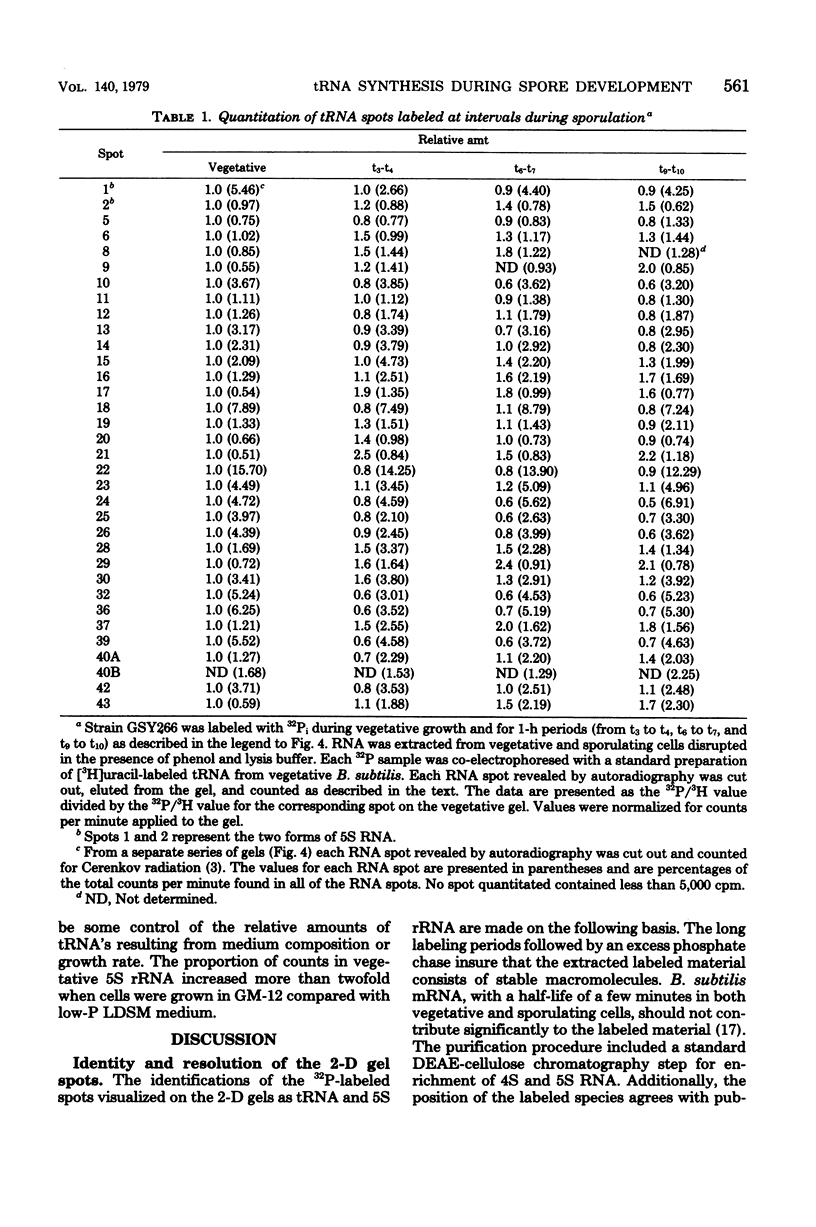
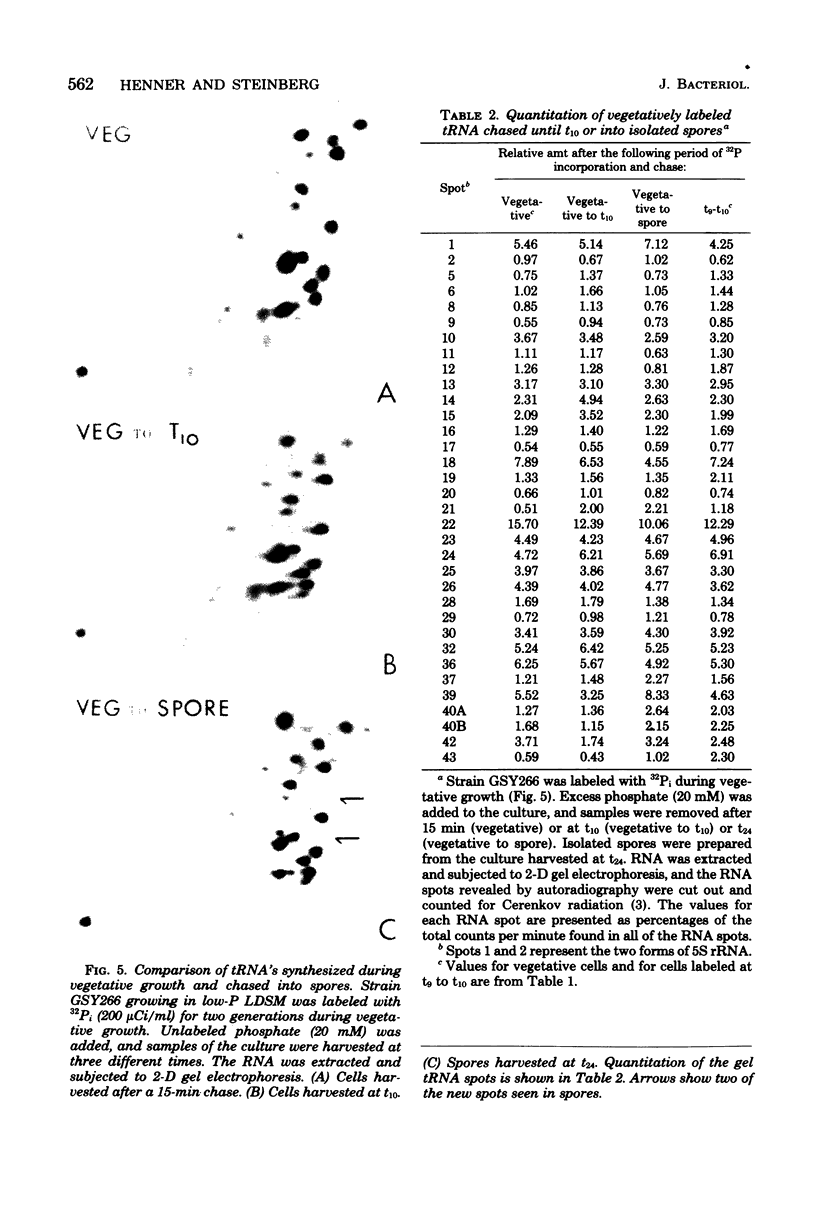
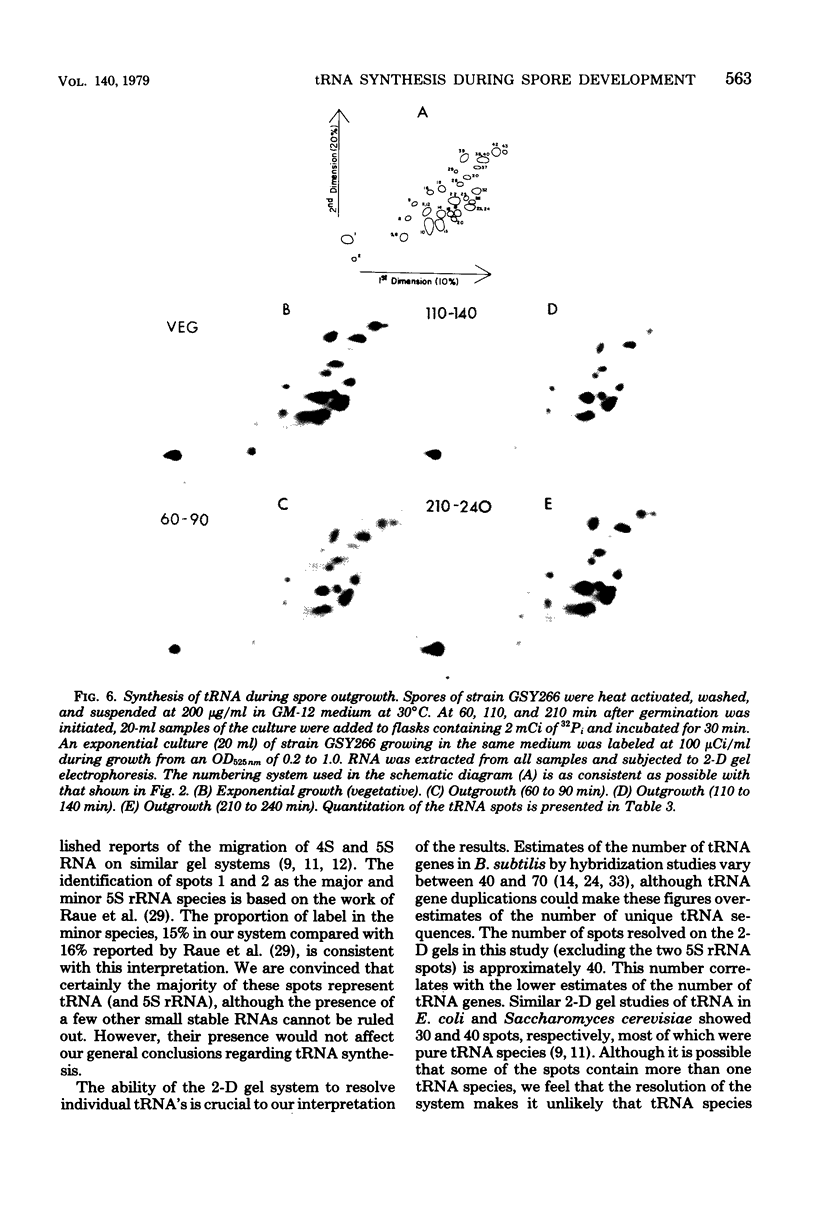
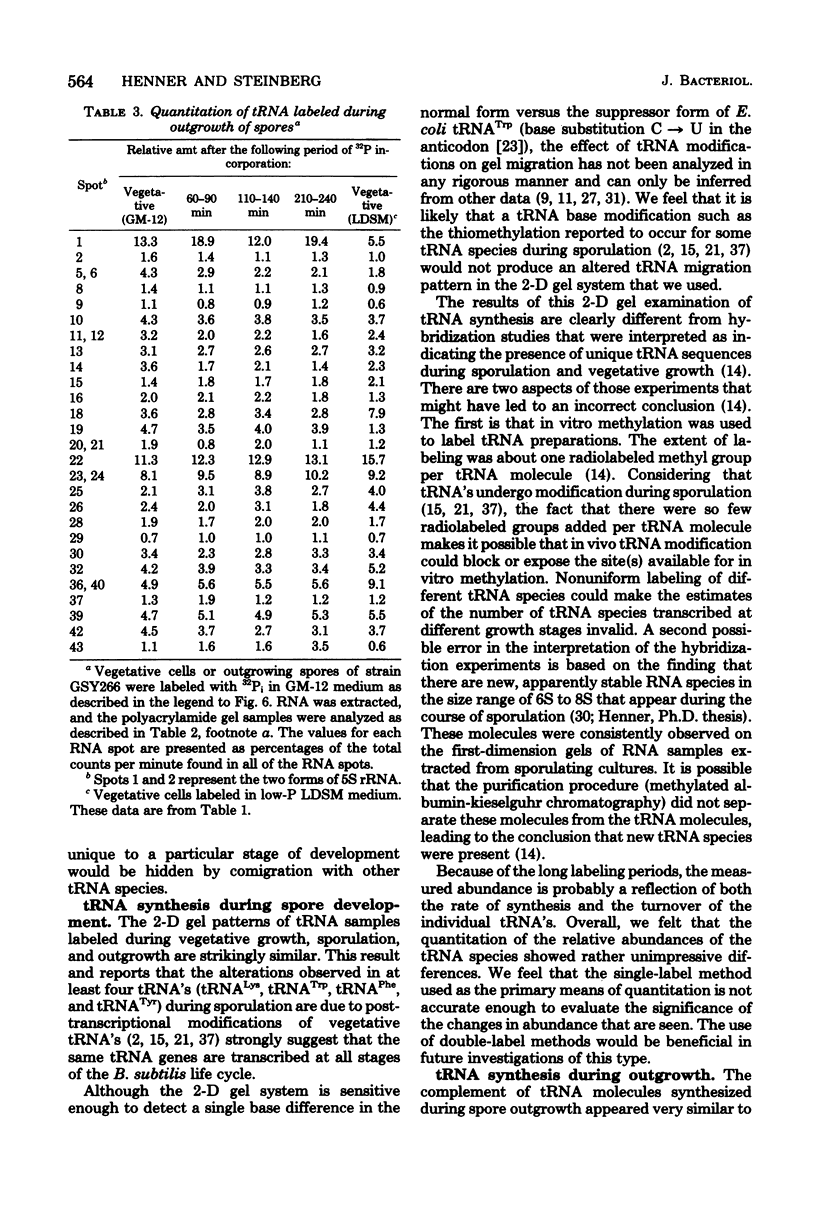
The synthesis of transfer ribonucleic acid (tRNA) was examined during spore formation and spore outgrowth in Bacillus subtilis by two-dimensional polyacrylamide gel electrophoresis of in vivo 32P-labeled RNA. The two-dimensional gel system separated the B. subtilis tRNA's into 32 well-resolved spots, with the relative abundances ranging from 0.9 to 17% of the total. There were several spots (five to six) resolved which were not quantitated due to their low abundance. All of the tRNA species resolved by this gel system were synthesized at every stage examined, including vegetative growth, different stages of sporulation, and different stages of outgrowth. Quantitation of the separated tRNA's showed that in general the tRNA species were present in approximately the same relative abundances at the different developmental periods. tRNA turnover and compartmentation occurring during sporulation were examined by labeling during vegetative growth followed by the addition of excess phosphate to block further 32P incorporation. The two-dimensional gels of these samples showed the same tRNA's seen during vegetative growth, and they were in approximately the same relative abundances, indicating minimal differences in the rates of turnover of individual tRNA's. Vegetatively labeled samples, chased with excess phosphate into mature spores, also showed all of the tRNA species seen during vegetative growth, but an additional five to six minor spots were also observed. These are hypothesized to arise from the loss of 3'-terminal residues from preexisting tRNA's.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. L., Sueoka N. Phase transitions in ribonucleic acid synthesis during germination of Bacillus subtilis spores. Proc Natl Acad Sci U S A. 1968 Jan;59(1):153–160. doi: 10.1073/pnas.59.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold H. H., Keith G. The nucleotide sequence of phenylalanine tRNA from Bacillus subtilis. Nucleic Acids Res. 1977 Aug;4(8):2821–2829. doi: 10.1093/nar/4.8.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUNSBERG H., GUYVER A. AUTOMATIC LIQUID SCINTILLATION COUNTING OF HIGH-ENERGY BETA-EMITTERS IN TISSUE SLICES AND AQUEOUS SOLUTIONS IN THE ABSENCE OF ORGANIC SCINTILLATOR. Anal Biochem. 1965 Jan;10:86–95. doi: 10.1016/0003-2697(65)90241-1. [DOI] [PubMed] [Google Scholar]
- BRUNNGRABER E. F. A simplified procedure for the preparation of "soluble" RNA from rat liver. Biochem Biophys Res Commun. 1962 Jun 19;8:1–3. doi: 10.1016/0006-291x(62)90223-1. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F., Barrell B. G. The sequence of 5 s ribosomal ribonucleic acid. J Mol Biol. 1968 Jun 28;34(3):379–412. doi: 10.1016/0022-2836(68)90168-x. [DOI] [PubMed] [Google Scholar]
- Doi R. H. Genetic control of sporulation. Annu Rev Genet. 1977;11:29–48. doi: 10.1146/annurev.ge.11.120177.000333. [DOI] [PubMed] [Google Scholar]
- Fradin A., Gruhl H., Feldmann H. Mapping of yeast tRNAs by two-dimensional electrophoresis on polyacrylamide gels. FEBS Lett. 1975 Feb 1;50(2):185–189. doi: 10.1016/0014-5793(75)80485-6. [DOI] [PubMed] [Google Scholar]
- Ginsburg D., Steitz J. A. The 30 S ribosomal precursor RNA from Escherichia coli. A primary transcript containing 23 S, 16 S, and 5 S sequences. J Biol Chem. 1975 Jul 25;250(14):5647–5654. [PubMed] [Google Scholar]
- Ikemura T., Dahlberg J. E. Small ribonucleic acids of Escherichia coli. I. Characterization by polyacrylamide gel electrophoresis and fingerprint analysis. J Biol Chem. 1973 Jul 25;248(14):5024–5032. [PubMed] [Google Scholar]
- Ikemura T., Shimura Y., Sakano H., Ozeki H. Precursor molecules of Escherichia coli transfer RNAs accumulated in a temperature-sensitive mutant. J Mol Biol. 1975 Jul 25;96(1):69–86. doi: 10.1016/0022-2836(75)90182-5. [DOI] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- Jeng Y. H., Doi R. H. New transfer ribonucleic acid species during sporulation of Bacillus subtilis. J Bacteriol. 1975 Mar;121(3):950–958. doi: 10.1128/jb.121.3.950-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith G., Rogg H., Dirheimer G., Menichi B., Heyham T. Post-transcriptional modification of tyrosine tRNA as a function of growth in Bacillus subtilis. FEBS Lett. 1976 Jan 15;61(2):120–123. doi: 10.1016/0014-5793(76)81017-4. [DOI] [PubMed] [Google Scholar]
- Kennett R. H., Sueoka N. Gene expression during outgrowth of Bacillus subtilis spores. The relationship between gene order on the chromosome and temporal sequence of enzyme synthesis. J Mol Biol. 1971 Aug 28;60(1):31–44. doi: 10.1016/0022-2836(71)90445-1. [DOI] [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- Littauer U. Z., Inouye H. Regulation of tRNA. Annu Rev Biochem. 1973;42:439–470. doi: 10.1146/annurev.bi.42.070173.002255. [DOI] [PubMed] [Google Scholar]
- Losick R., Pero J. Bacillus subtilis RNA polymerase and its modification in sporulating and phage-infected bacteria. Adv Enzymol Relat Areas Mol Biol. 1976;44:165–185. doi: 10.1002/9780470122891.ch5. [DOI] [PubMed] [Google Scholar]
- Menichi B., Heyman T. Study of tyrosine transfer ribonucleic acid modification in relation to sporulation in Bacillus subtilis. J Bacteriol. 1976 Jul;127(1):268–280. doi: 10.1128/jb.127.1.268-280.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
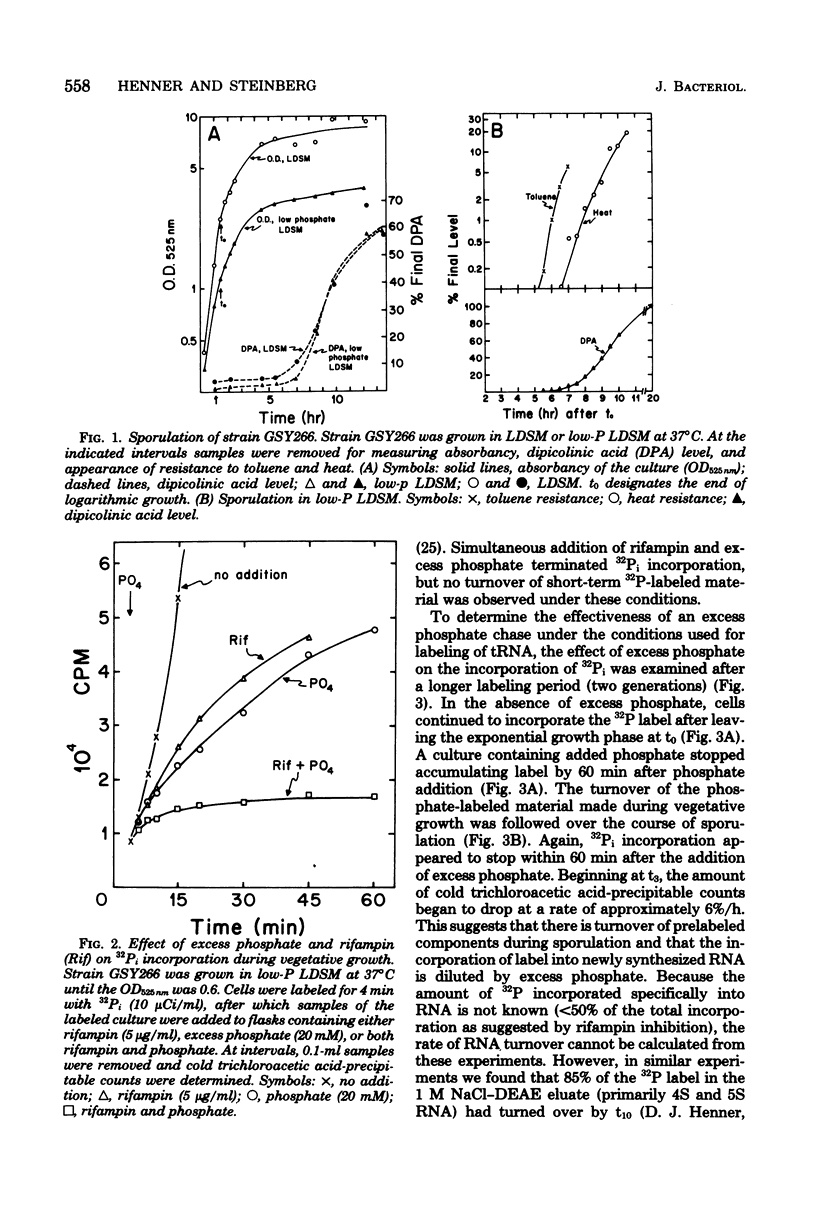
- Milhaud P., Balassa G. Biochemical genetics of bacterial sporulation. IV. Sequential development of resistances to chemical and physical agents during sporulation of Bacillus subtilis. Mol Gen Genet. 1973 Sep 12;125(3):241–250. doi: 10.1007/BF00270746. [DOI] [PubMed] [Google Scholar]
- Morgan E. A., Nomura M. Deletion analysis of the expression of rRNA genes and associated tRNA genes carried by a lambda transducing bacteriophage. J Bacteriol. 1979 Jan;137(1):507–516. doi: 10.1128/jb.137.1.507-516.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M., Oishi A., Sueoka N. Location of genetic loci of soluble RNA on Bacillus subtilis chromosome. Proc Natl Acad Sci U S A. 1966 May;55(5):1095–1103. doi: 10.1073/pnas.55.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace B., Peterson R. L., Pace N. R. Formation of all stable RNA species in Escherichia coli by posttranscriptional modification. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1097–1104. doi: 10.1073/pnas.65.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieczenik G., Barrell B. G., Gefter M. L. Bacteriophage phi 80-induced low molecular weight RNA. Arch Biochem Biophys. 1972 Sep;152(1):152–165. doi: 10.1016/0003-9861(72)90203-2. [DOI] [PubMed] [Google Scholar]
- Raué H. A., Rosner A., Planta R. J. Heterogeneity of the genes coding for 5 S RNA in three related strains of the genus Bacillus. Mol Gen Genet. 1977 Nov 14;156(2):185–193. doi: 10.1007/BF00283491. [DOI] [PubMed] [Google Scholar]
- Segall J., Losick R. Cloned Bacillus subtilis DNA containing a gene that is activated early during sporulation. Cell. 1977 Aug;11(4):751–761. doi: 10.1016/0092-8674(77)90289-6. [DOI] [PubMed] [Google Scholar]
- Sehulster L. M., Varricchio F., Raska K Jun Synthesis of transfer ribonucleic acid in KB cells infected with adenovirus type 2. J Gen Virol. 1978 Jul;40(1):183–194. doi: 10.1099/0022-1317-40-1-183. [DOI] [PubMed] [Google Scholar]
- Setlow P., Primus G., Deutscher M. P. Absence of 3'-terminal residues from transfer ribonucleic acid of dormant spores of Bacillus megaterium. J Bacteriol. 1974 Jan;117(1):126–132. doi: 10.1128/jb.117.1.126-132.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I., Dubnau D., Morrell P., Marmur J. Chromosomal location of DNA base sequences complementary to transfer RNA and to 5 s, 16 s and 23 s ribosomal RNA in Bacillus subtilis. J Mol Biol. 1968 Apr 14;33(1):123–140. doi: 10.1016/0022-2836(68)90285-4. [DOI] [PubMed] [Google Scholar]
- Steinberg W. Thermal death of temperature-sensitive lysyl- and tryptophanyl-transfer ribonucleic acid synthetase mutants of Bacillus subtilis: effect of culture medium and developmental stage. J Bacteriol. 1974 Nov;120(2):767–778. doi: 10.1128/jb.120.2.767-778.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vold B. S. Analysis of isoaccepting transfer ribonucleic acid species of Bacillus subtilis: changes in chromatography of transfer ribonucleic acids associated with stage of development. J Bacteriol. 1973 Apr;114(1):178–182. doi: 10.1128/jb.114.1.178-182.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vold B. S. Post-transcriptional modifications of the anticodon loop region: alterations in isoaccepting species of tRNA's during development in Bacillus subtilis. J Bacteriol. 1978 Jul;135(1):124–132. doi: 10.1128/jb.135.1.124-132.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vold B. Degree of completion of 3'-terminus of transfer ribonucleic acids of Bacillus subtilis 168 at various developmental stages and asporogenous mutants. J Bacteriol. 1974 Mar;117(3):1361–1362. doi: 10.1128/jb.117.3.1361-1362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E. C., Steinberg W. The effect of gene position, gene dosage and a regulatory mutation on the temporal sequence of enzyme synthesis accompanying outgrowth of Bacillus subtilis spores. Mol Gen Genet. 1978 Jan 17;158(3):287–296. doi: 10.1007/BF00267200. [DOI] [PubMed] [Google Scholar]